
Systematic use of point of care ultrasound in neurosurgical intensive care unit: a practical approach
Introduction
Bedside ultrasonography (USG)/sonoscopy procedures, also known as “Point Of Care Ultrasound” (POCUS), is a cost-effective, non-invasive procedure which provides real time information and is more specific in diagnostic and therapeutic utilities (1). POCUS has been shown to be a superior imaging option than conventional imaging technique in emergency and critical care setting by minimizing mobilization, reducing cost, assist in invasive procedures and assess the condition in real time (1-3). Various authors have used POCUS in different ways for independent brain pathologies (4-11). However, it is not being routinely used in neurosurgical intensive care unit (ICU) due to steep learning curve and confusion with which modality to use. POCUS being problem oriented rather than organ guided approach has been felt to have minimal use in neurosurgical ICU.
POCUS hands-on courses were commenced in Kathmandu Medical College Teaching Hospital (KMCTH) (1st course was held in 2014) (12). Understanding the aforementioned concern and confusion related to POCUS, we introduced a systematic use of sonoscopy in critically ill patients in our neurosurgical ICU to not only perform and record POCUS modalities systematically but also improve communication, record keeping and training. We studied the various indications and benefits of using the sonoscopy and hereby providing a practical layout of the procedure.
The objective of this study is to know indications and methods used for POCUS based procedures; to provide a general description of these bedside procedures and to evaluate its effectiveness in changing the management in a neurosurgery ICU setting. We present the following article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-667/rc).
Methods
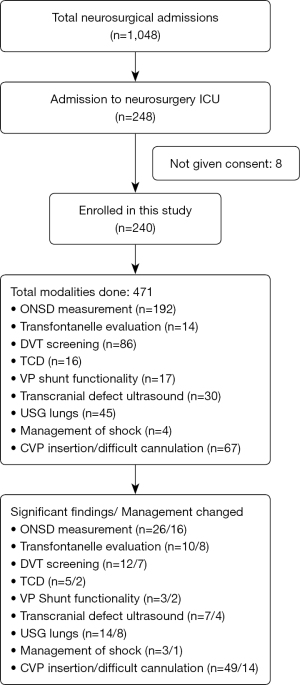
This is a prospective, single center cohort observational study done in patients who were admitted in neurosurgical ICU of KMCTH over one year (17th September 2020 to 16th September 2021). POCUS was used daily as per a standardized format sheet (Figure 1) for multiple purposes for the management of various neurosurgical and non-neurosurgical conditions. The format was comprehensive to include all possible modalities applicable in ICU setting, however some were to be performed based on the indications and patient’s scenario. The findings or interventions were recorded on the format sheet. The procedures mentioned in the standardized format helped guide the operator to perform the procedures in an order.

POCUS was used for primary resuscitation, daily secondary surveys including optic nerve sheath diameter (ONSD) measurement, trans-fontanelle evaluation, transcranial Doppler (TCD), screening for deep vein thrombosis (DVT), ventriculo-peritoneal (VP) shunt functionality and transcranial defect ultrasound and focused ultrasound chest and evaluation of hemodynamic shock. POCUS was used to place central venous lines, difficult cannulation, pleural and trans-fontanelle tapping.
Residents on duty were formally trained and asked to perform at least 10 studies or procedures under supervision before independently performing the examinations. Standardized method of evaluation and intervention was used. Outcome was studied to know the impact of the POCUS and difficulties encountered during its use.
Statistical analyses were performed with SPSS 23.0 (SPSS, Inc., Chicago, IL. USA). Pearson Chi square was used to check for significance with P<0.05.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of KMCTH (No. 04082022/05) and informed consent was taken from all the patients and patients’ parents or legal guardians.
Technique used
ONSD measurement
Increase in intracranial pressure (ICP) leads to papilledema where cerebrospinal fluid (CSF) collects arounds optic nerve limited by duramater, distending the optic disc. The resulting expansion of ONSD suggests increase in ICP. ONSD measurement is indicated in patients with any intracranial pathologies that can increase ICP in turn rising ONSD values.
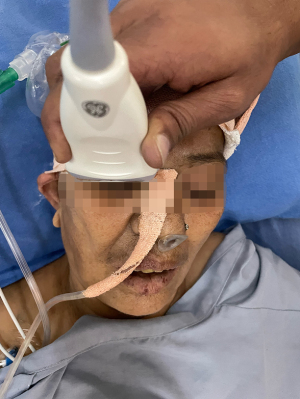
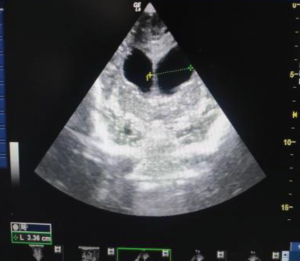
ONSD is measured by using linear probe of the ultrasound in B mode with patient in supine position with the examiner at the head of the examination table with head elevated for 20°–30°. The ideal situation would be to instruct the patient to look straight with eyes closed without moving the eyeball. However, in ICU settings we place the probe with gel over the upper eyelid with wrist resting on forehead to avoid putting pressure on the eye (Figure 2). The optic nerve is visualized posterior to the globe in axial plane. Once optic nerve and surrounding sheath is visualized, freeze button is clicked and ONSD measurement is taken 3 mm behind the globe perpendicular to the optic nerve axis (Figure 3).
Trans-fontanelle evaluation
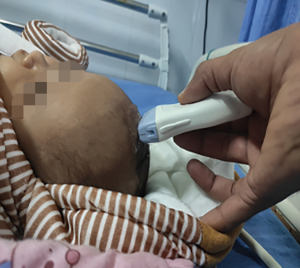
Trans-fontanelle evaluation of the head is done for neonates through the open anterior fontanelle for intracranial or spinal pathologies. Curvilinear probe of the ultrasound is used to assess the fontanelle opening in B mode to look for the size of lateral ventricle. The head of the child is held by an assistant, gel applied and trans-fontanelle POCUS is done. To look for the coronal image, the probe is angled to depict the frontal horns of the lateral ventricles.
Trans-fontanelle tapping of CSF can be done in case of distended ventricles. Under ultrasound guidance, a butterfly cannula is inserted into the frontal horn of lateral ventricle using aseptic measures. CSF is then allowed to flow out. Sterile dressing is applied at the puncture site (Figures 4-6).
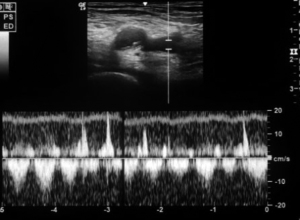
TCD USG
TCD USG is being used to study cerebral hemodynamics for the evaluation of stenosis, vasospasm and occlusion of cerebral arteries; mainly done after an aneurysm clipping surgery.
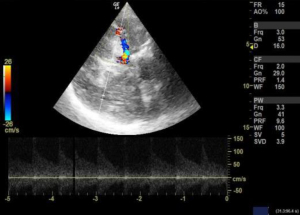
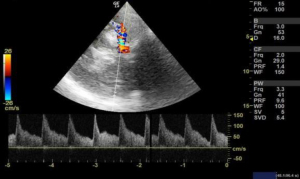
We used 3 MHz Doppler probe in Doppler and color mode for the evaluation of blood flow signals mainly in the anterior, middle and posterior cerebral arteries and internal carotid arteries through a thin temporal window directing the probe towards contralateral side. Blood flow velocity is then measured in cm/s for the arteries of interest. When evaluating the different arteries, the depth of the artery and the color that the arteries exhibit were used to identify different arteries. Lindegaard ratio was then calculated by dividing the mean velocity of middle cerebral artery to that of internal carotid artery, which helped to differentiate hyperemia from true vasospasm (Figures 7,8).
Transcranial defect ultrasound
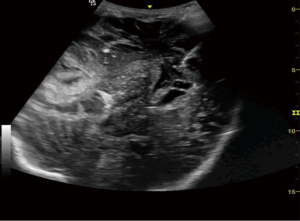
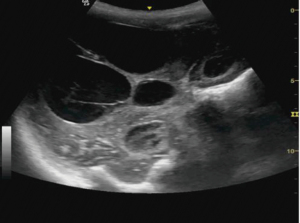
Hemicraniectomy provides a wide window for intracranial imaging with images comparable to magnetic resonance imaging (MRI) and computed tomography (CT) scan. Transcranial defect ultrasound was used to look for the ventricle size, size of hematoma or collections, midline shift and brain stem in patients who have undergone hemicraniectomy after trauma to the head. Curvilinear probe was used for the evaluation in B mode. Gel was applied over the hemicraniectomy side and the probe is rotated to look for the above-mentioned pathology in real time (Figures 9-11).
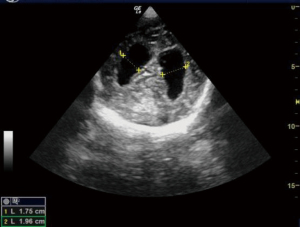
VP shunt functionality
We used ultrasound to check for VP shunt patency and whether the shunt is functional or not for patients after a VP shunt surgery. After localizing the shunt by palpation, a linear ultrasound probe was kept over the tube just distal to shunt chamber. M mode was turned on. The shunt chamber was then pressed and to check whether there was a flow in the tube which can be visualized in M mode. Ultrasound abdomen was also done to look for free fluid in the peritoneal cavity which indirectly suggested flow of CSF through VP shunt into peritoneal cavity (Figure 12).
Ultrasound lungs
As patients admitted to neurosurgical ICU are at risk of developing ventilator associated pneumonia, we find daily lung ultrasound screening a useful alternative to chest X-ray (CXR) or CT chest. Besides we looked for pneumothorax, pleural collection and pleural effusion that needed immediate management in patients with an ICU stay of >3 days.
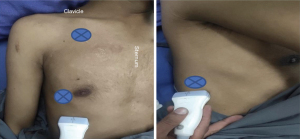
We used a 5 MHz curvilinear probe and evaluated the lung zone according to the blue points (bedside lung ultrasound in emergency, BLUE protocol) as shown in Figure 13. Upper BLUE point is at 1 cm below the clavicle at the midclavicular line. Lower BLUE point is lateral to the nipple in the anterior axillary line. The posterolateral point lies in the intersection of posterior axillary line and horizontal line along the nipple. The probe can be moved in any direction in order to get more information. The point of interest is the chest wall, pleural space, lung parenchyma and the diaphragm.
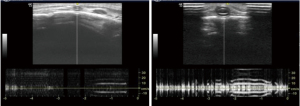
As air generates artifacts during lung ultrasound, knowledge of the normal pattern is needed to perform and interpret the results. There is a narrow window for ultrasound between the ribs which casts posterior acoustic shadow. At around 1 cm below the skin, we can appreciate a hyperechoic pleura which slides forward and backwards with respiration and a ‘bat sign’ (pleural line in between two ribs) as shown in Figure 9 which is a normal finding. We then used the M-mode (time motion) to check for either a normal ‘seashore sign’ (Figure 14) or a ‘barcode sign’ to diagnose pneumothorax (Figure 15). Then the probe is kept at zone 2 and checked for the same. Probe is then placed at zone 3 and looked for any collections. Appreciating a hypoechoic or anechoic homogenous collection is suggestive of collection or pleural effusion (depending upon the amount of collection) in the dependent part of the thoracic cavity above the diaphragm. A syringe is taken to aspirate pleural fluid under ultrasound guidance.
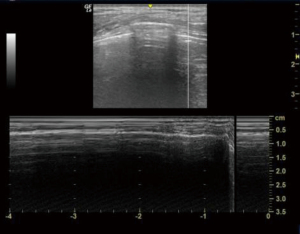
Screening for DVT
Neurosurgical ICU patients are at a risk of developing DVT as patients are bed ridden. Ultrasound can be used to screen these ICU patients for DVT. Although a proper DVT screening has to scan common femoral, great saphenous, superficial femoral, deep femoral, popliteal, posterior tibial, and peroneal veins; this can be time consuming in ICU settings. So, we looked for compressibility and collapse of common femoral and popliteal veins only (Figures 16,17).
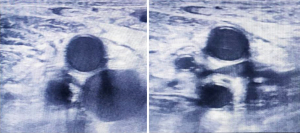
Linear probe was kept at the calf and groin on both sides respectively. M mode was used to check for the flow in the veins.
Other scans
Inferior vena cava (IVC) diameter was measured in supine position by placing a 5 MHz probe in subxiphoid location in sagittal section 2 cm proximal to junction of hepatic vein and IVC and diameter was measured in M mode. IVC diameter can be useful to check if a patient has deteriorated cause of hypovolemia or fluid overload. Ultrasound was also used for other purpose like central venous catheter placement, difficult cannulation, arterial line placement, urinary bladder scan and e-FAST scan.
Results
A total of 240 neurosurgical ICU admissions were studied after being taking consent. The number of patients enrolled according to the underlying disease is given in Table 1. The use of standardized format helped maintain homogeneity of protocols and was found to be good teaching tool. It also served to communicate efficiently. Different modalities of ultrasound as mentioned in the standardized format were used for all ICU patients according to the protocol (Figure 1), however the modality/type of intervention performed depend upon the need of the patient (Figure 1).
Table 1
| Underlying disease | Number of patients screened |
|---|---|
| Traumatic brain/spine pathologies | 99 |
| Spontaneous hemorrhage/ischemic stroke | 66 |
| Brain tumor | 26 |
| Aneurysm | 16 |
| Meningomyocele | 14 |
| Brain infection | 14 |
| Other brain pathologies | 5 |
All patients in whom POCUS detected an abnormal finding, formal investigations/definitive procedure was performed. The number of significant findings and interventions done for each modality assessed is shown in Figure 18. All the images were saved in the sonoscopy machine, which was then reviewed for accuracy of the finding and result and corrections to maintain a required standard for the study. Routine screening and follow up of all enrolled patients were done according to the protocol till the patients were shifted from neurosurgical ICU.
Trans-fontanelle tapping was done in neonate with hydrocephalus under ultrasound guidance and around 10 to 30 mL of CSF was aspirated according to the size of ventricle. Similarly, pleural tapping was done for a patient with a massive hemothorax. POCUS was used for placement of central venous catheters, arterial line placement as well as in difficult cannulation.
Out of the 240 neurosurgical ICU admissions, modalities mentioned above were used for 471 times. The age of patients ranged from 3 days to 87 years, with 63.7% male. Single modality of POCUS was used in 27.6%, while two or three modalities were used in 29.7% each (Figure 18).
POCUS allowed for timely diagnosis and of significant findings in 62 patients (25.83%) (Figure 18). This has led to effective change in management in 48.1% as compared to 11.1% cases in which POCUS failed to identify significant findings (which were picked on other radiologcial investigations) who later required intervention (χ2=30.7909, P<0.001) (Table 2). None of the patients had any adverse effect after being enrolled in this study.
Table 2
| Significant finding on POCUS examination | Intervention | Total (P<0.001) | |
|---|---|---|---|
| Not required | Performed | ||
| Absent | 95 | 16 | 111 |
| Present | 67 | 62 | 129 |
| Total | 162 | 78 | 240 |
POCUS, point of care ultrasound.
Discussion
POCUS is a non-invasive procedure, which is becoming an integral part in care and management of patients as it provides a real time information. It is also helpful in assessing progress of the disease and response to specific treatment (13). POCUS can be done bedside without mobilizing patient to minimize risk, reducing cost and monitoring complications. It also helps in invasive procedures that are done in neurosurgical ICU (14).
The four pillars during bedside physical examination are to inspect, palpate, percuss, and auscultate. Incorporating appropriate technology (bedside ultrasound or POCUS) can not only improve the performance but also assist in superior decision making. Thus, making bedside ultrasound the fifth pillar of physical examination (15).
We have described our systematic approach to use of POCUS in our neurosurgical ICU. This has maintained uniformity and proved to be a good teaching tool. In almost half of the cases, where significant findings were noted, we were able to perform timely intervention.
Of all the modalities, ONSD was most commonly used in our ICU. ONSD measurement is a simple, non-invasive procedure with high diagnostic accuracy for intracranial hypertension. Maissan et al. stated that if ONSD >5.0 mm; ICP being >20 mmHg is 94% sensitive and 98% specific (16). As the intracranial CSF spaces are a continuum with optic nerve subarachnoid spaces; any acute or chronic disease with increased ICP can lead to the increase in ONSD. In this study, two or more values of ONSD >6 mm was considered a significant finding.
Many authors have described several intracranial windows for ultrasound, but they are rarely performed (6). Transcranial POCUS in a hemicraniectomy patient provides a unique window for evaluating various pathologies which has been reported to identify important intracranial pathologies (13,17). Although, a B mode ultrasound can be done in patients with intact skull, the visibility becomes far better when the bone flap is removed. In this study, size of ventricles, hematoma or collections and midline shift was evaluated in serial monitoring to monitor the underlying intracranial pathologies. Transtemporal windows is another commonly performed window for the evaluation of vasospasm of cerebral arteries. Owing to an open fontanelle and thinner transtemporal window in neonates and infant, the imaging has shown to be highly sensitive and specific to that of an adult hemicraniectomy patient (6). Although, authors have often reported the window to difficult, time consuming and inadequate in adults (18), TCD in aneurysmal subarachnoid hemorrhage, however, has shown to be an important modality for monitoring of vasospasm (8). Lindegaard ratio of <3 is considered normal with values of 3.0–4.5, 4.5–6 and >6 indicative of mild, moderate and severe vasospasm respectively (5).
Trans-fontanelle ultrasound outstand many clinical situations benefitting the patients that requires sedation in an unexpensive and non-invasive way (10). Recently [2020], The American Institute of Ultrasound in Medicine (AIUM) has published a consensus for cranial ultrasound in infants for various cranial conditions and monitor hydrocephalus (19), highlighting ultrasound being an extremely valuable tool. Trans-fontanelle taping has been described in literature done in either brain abscess (11) or in subdural empyema (7). In this study, serial monitoring of the size of ventricles were done to monitor for hydrocephalus. We used taping to send CSF culture or in neonates who required shunting but parental refused to consent.
Using ultrasound to check for the functionality of VP shunt has not been reported in the literature. Using ultrasound to check for the blockage or a non-functional VP shunt including presence or absence of collection in the abdomen can be helpful in the management of VP shunt.
There are adequate papers published highlighting the importance of lung POCUS in emergency as well as in ICUs (1-3,15). It has shown to be a good alternative to thoracic CT and CXR to diagnose common lung pathologies (2). Out of many lung ultrasound protocols, this study uses the BLUE protocol in neurosurgical ICU setting (20). A standardized thoracic point in BLUE protocol is adapted in this study which gives a uniform, accurate findings. Additionally, it is easy to perform because all life threating conditions of the chest are superficial. This study only focused on pneumothorax, pleural collection and pleural effusion which are more common in neurosurgical ICUs.
A complete DVT screening including both the lower extremities and the calf veins is time consuming (21), especially in case of neurosurgical ICUs. Compression testing of the proximal veins is sufficient for evaluation of DVT (22) , with authors claiming a diagnostic accuracy of around 95% (1). In this study, 8.1% of DVT screened patients had a positive POCUS findings which was corelated with repeated DVT scan and managed accordingly.
Out of many roles of POCUS, evaluation of hemodynamic shock is also one of the important techniques in neurosurgical ICU to know the fluid status. Aggressive fluid resuscitation can be detrimental for patients in shock. Measurement of IVC diameter helps in the detection of hypovolemic shock as well as following up on hypovolemic patients and responding to fluid challenge (23). Although neurosurgical ICU patients have central venous pressure (CVP) catheter inserted, studies have shown CVP should not be used to guide clinical decisions regarding volume management (24,25). As IVC diameter is minimally affected by compensatory response of body to fluid loss, it is more reflective than other arterial parameters such as blood pressure or pulse rate (26).
There are many studies describing new approaches for diagnosing different neurosurgical condition, however due to lack of proper evidence, further studies are required. In this study, only the POCUS modality useful in neurosurgery ICU has been described which helped in patient management and to guide the treatment. It was used in primary resuscitation for evaluation of chest and evaluation of hemodynamic shock in life threatening emergency conditions; and in daily secondary surveys including ONSD measurement, trans-fontanelle evaluation, TCD, DVT screening, VP shunt functionality and transcranial defect ultrasound.
Although, POCUS in neurosurgical ICUs can never replace gold standard imaging modalities, it can be a useful tool to increase potential ability while using fewer resources (6). POCUS is becoming increasingly popular, with many authors strongly suggesting to add POCUS to the ICUs (17) and to train the neurosurgical residents eventually leading to the betterment of patient care.
Challenges and limitations
There was an initial lag in adopting the technique to be familiar with the imaging appearance. Although POCUS has an operator dependent image acquisition; the team developed confidence by teaching and learning modality, leading to accurate results and reduced time taken to perform the procedure. Poor sonography images, interpretation of results, inter-observer differences, human resource management and adapting to the ultrasound machine were some of the difficulties faced during this study. The diagnostic reasoning, judging a diagnostic impression or conforming a specific hypothesis could have led to cognitive, anchoring or conformation bias.
Conclusions
Routine systematic use of ultrasound can be beneficial not only for rapid diagnosis and prompt management of patients, but also to perform various procedures in neurosurgery ICU. POCUS has also changed the paradigm in patient management by decreasing complications associated with various procedures performed in neurosurgery ICU. Incorporating POCUS into critical care training is increasing because it is beneficial for both the patients and the treating physician.
Acknowledgments
We thank the residents and doctors of Department of Neurosurgery who helped us in this work.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-667/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-667/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of KMCTH (No. 04082022/05) and informed consent was taken from all the patients and patients’ parents or legal guardians.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Campbell SJ, Bechara R, Islam S. Point-of-Care Ultrasound in the Intensive Care Unit. Clin Chest Med 2018;39:79-97. [Crossref] [PubMed]
- Shrestha GS, Weeratunga D, Baker K. Point-of-Care Lung Ultrasound in Critically ill Patients. Rev Recent Clin Trials 2018;13:15-26. [Crossref] [PubMed]
- Lichtenstein D, van Hooland S, Elbers P, Malbrain ML. Ten good reasons to practice ultrasound in critical care. Anaesthesiol Intensive Ther 2014;46:323-35. [Crossref] [PubMed]
- Robba C, Goffi A, Geeraerts T, Cardim D, Via G, Czosnyka M, Park S, Sarwal A, Padayachy L, Rasulo F, Citerio G. Brain ultrasonography: methodology, basic and advanced principles and clinical applications. A narrative review. Intensive Care Med 2019;45:913-27. [Crossref] [PubMed]
- Loomis AL, Chakko MN. Doppler Trans-Cranial Assessment, Protocols, And Interpretation. In: StatPearls. Treasure Island, FL, USA: StatPearls Publishing, 2022.
- Shah A, Oliva C, Barnes R, Presley B. Identification of intracranial hemorrhage progression by transcranial point-of-care ultrasound in a patient with prior hemicraniectomy: a case report. J Ultrasound 2022;25:399-402. [Crossref] [PubMed]
- Kanu OO, Nnoli C, Olowoyeye O, Ojo O, Esezobor C, Adeyomoye A, Bankole O, Asoegwu C, Temiye E. Infantile subdural empyema: The role of brain sonography and percutaneous subdural tapping in a resource-challenged region. J Neurosci Rural Pract 2014;5:355-9. [Crossref] [PubMed]
- Hakimi R, Alexandrov AV, Garami Z. Neuro-ultrasonography. Neurol Clin 2020;38:215-29. [Crossref] [PubMed]
- Lochner P, Czosnyka M, Naldi A, Lyros E, Pelosi P, Mathur S, Fassbender K, Robba C. Optic nerve sheath diameter: present and future perspectives for neurologists and critical care physicians. Neurol Sci 2019;40:2447-57. [Crossref] [PubMed]
- Llorens-Salvador R, Moreno-Flores A. The ABCs of transfontanellar ultrasound and more. Radiologia 2016;58:129-41. [Crossref] [PubMed]
- Theophilo F, Burnett A, Jucá Filho G, Adler A, Miranda S, Theophilo L, Carvalho M, Lopes J. Ultrasound-guided brain abscess aspiration in neonates. Childs Nerv Syst 1987;3:371-4. [Crossref] [PubMed]
- Thapa A. Need of Integrating Sonoscopy in Undergraduate Medical Education in Developing Countries. J Nepal Health Res Counc 2020;18:556-9. [Crossref] [PubMed]
- Sarwal A, Elder NM. Point-of-care Cranial Ultrasound in a Hemicraniectomy Patient. Clin Pract Cases Emerg Med 2018;2:375-7. [Crossref] [PubMed]
- Bilotta F, Dei Giudici L, Lam A, Rosa G. Ultrasound-based imaging in neurocritical care patients: a review of clinical applications. Neurol Res 2013;35:149-58. [Crossref] [PubMed]
- Narula J, Chandrashekhar Y, Braunwald E. Time to Add a Fifth Pillar to Bedside Physical Examination: Inspection, Palpation, Percussion, Auscultation, and Insonation. JAMA Cardiol 2018;3:346-50. [Crossref] [PubMed]
- Maissan IM, Dirven PJ, Haitsma IK, Hoeks SE, Gommers D, Stolker RJ. Ultrasonographic measured optic nerve sheath diameter as an accurate and quick monitor for changes in intracranial pressure. J Neurosurg 2015;123:743-7. [Crossref] [PubMed]
- Srinivasan V, Smith M, Bonomo J. Bedside Cranial Ultrasonography in Patients with Hemicraniectomies: A Novel Window into Pathology. Neurocrit Care 2019;31:432-3. [Crossref] [PubMed]
- Marinoni M, Ginanneschi A, Forleo P, Amaducci L. Technical limits in transcranial Doppler recording: inadequate acoustic windows. Ultrasound Med Biol 1997;23:1275-7. [Crossref] [PubMed]
- AIUM Practice Parameter for the Performance of Neurosonography in Neonates and Infants. J Ultrasound Med 2020;39:E57-61. [PubMed]
- Lichtenstein DA. BLUE-protocol and FALLS-protocol: two applications of lung ultrasound in the critically ill. Chest 2015;147:1659-70. [Crossref] [PubMed]
- Swanson E. Ultrasound screening for deep venous thrombosis detection: a prospective evaluation of 200 plastic surgery outpatients. Plast Reconstr Surg Glob Open 2015;3:e332. [Crossref] [PubMed]
- Heijboer H, Büller HR, Lensing AW, Turpie AG, Colly LP, ten Cate JW. A comparison of real-time compression ultrasonography with impedance plethysmography for the diagnosis of deep-vein thrombosis in symptomatic outpatients. N Engl J Med 1993;329:1365-9. [Crossref] [PubMed]
- Zengin S, Al B, Genc S, Yildirim C, Ercan S, Dogan M, Altunbas G. Role of inferior vena cava and right ventricular diameter in assessment of volume status: a comparative study: ultrasound and hypovolemia. Am J Emerg Med 2013;31:763-7. [Crossref] [PubMed]
- Marik PE, Baram M, Vahid B. Does central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest 2008;134:172-8. [Crossref] [PubMed]
- De Backer D, Vincent JL. Should we measure the central venous pressure to guide fluid management? Ten answers to 10 questions. Crit Care 2018;22:43. [Crossref] [PubMed]
- Dipti A, Soucy Z, Surana A, Chandra S. Role of inferior vena cava diameter in assessment of volume status: a meta-analysis. Am J Emerg Med 2012;30:1414-9.e1. [Crossref] [PubMed]




