
Leadless pacemaker implantation and azygos continuation in the inferior vena cava: a case description
Introduction
Azygos continuation (AC) of the inferior vena cava (IVC), also known as the absence of the hepatic segment of the IVC with AC, is a rare anatomic variant in the general population with an incidence of 0.6% (1). AC of the IVC is congenital and independent from other anatomical variants. It is primarily caused by the absence or hypoplasia of the IVC’s hepatic segment. The IVC below the hepatic segments flows upward through the azygos into the superior vena cava (SVC) and eventually drains into the right atrium. The renal portion of the IVC receives blood flow from the kidneys and lower extremities and drains into the SVC through the azygos vein. The azygos vein, azygos arch, and SVC dilate to accommodate the increase in blood flow (2). Usually, AC in the IVC is asymptomatic and does not affect the functionality of the cardiovascular system. However, it significantly impacts leadless pacemaker (LP) implantation via the femoral vein. The LP is a feasible alternative to the single-ventricle pacemaker; that is, a pacemaker that simply paces the ventricle but not the atrium. LP is a novel technique that differs from traditional pacemakers in terms of the electronic components, implantation procedure, possible complications, and postoperative management. Unlike the conventional pacemaker implantation, where the lead is delivered to the heart by puncturing the subclavian veins to approach the SVC, the LP implantation involves puncturing the femoral vein and delivering the LP to the heart via the IVC (3). If a patient has AC of the IVC, an LP cannot be implanted through the IVC pathway or could be delivered to the wrong location, such as the SVC. Furthermore, routine preoperative examinations (e.g., cardiac ultrasound and chest X-ray) are ineffective in detecting AC. Herein, we report a case of AC of the IVC observed during LP implantation, which resulted in the abandonment of this procedure. Electrocardiogram (ECG)-gated computed tomography (CT) venography was used to identify the anatomic variant of the IVC as an AC. This patient eventually underwent conventional pacemaker implantation via the SVC.
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
A 50-year-old female patient was admitted to The Second Affiliated Hospital of Zhejiang University School of Medicine with a slow heart rate associated with chest tightness, palpitation, and weakness in the limbs for more than 10 years and a recent aggravation of the symptoms. The dynamic ECG indicated sinus arrest with a maximum interval of 3.38 seconds and persistent atrial flutter. Therefore, atrio-ventricular (AV) synchrony with a dual-chamber pacemaker was preferred, and the implantation of a single-chamber permanent pacemaker was acceptable. The patient’s preoperative cardiac ultrasound and digital chest X-ray revealed no significant abnormalities (Figure 1). She underwent digital subtraction venography (DSV) fluoroscopy the next day for the LP implantation. She chose the LP implantation because of aesthetic considerations and she had rejected the pacemaker pocket and transvenous lead. The type of pacemaker she selected was the Micra transcatheter pacing system (Micra-TPS, Medtronic Inc., Fridley, MN, USA). This pacemaker is a single-chamber ventricular pacemaker with an accelerometer-based rate-adaptive pacing and automated pacing capture threshold management to maximize battery longevity. When the digital subtraction angiography (DSA) was performed, the device was located in a steerable catheter delivery system with an outer diameter of 27-F. The device was inserted through the femoral vein toward the right ventricle via the IVC. However, as the catheter was delivered upward along the assumed right femoral vein–right iliac vein–IVC pathway, the delivery catheter entered the SVC, suggesting a malformation of the IVC. As a result, the LP implantation was abandoned.
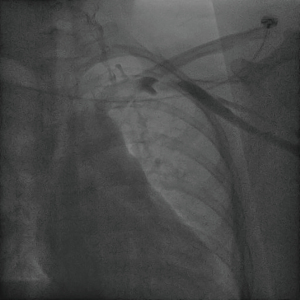
The patient underwent a DSA fluoroscopy for SVC venography the next day. The contrast agent flowed from the left axillary vein through the left subclavian vein into the left SVC, indicating a normal venous channel (Figure 2).
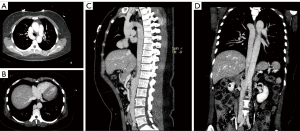
On the third postoperative day, the patient underwent an ECG-gated contrast-enhanced CT venography of the chest and abdomen on a dual-source Somatom Force 192-layer machine (Siemens, Erlangen, Germany; tube voltage: 80 kV, 250 mAs; pitch: 1; collimator width: 0.6 mm). The scan was performed in the arterial, portal, and venous phases. The images were sent to a workstation for postprocessing and 3-dimensional reconstruction.
The venography results revealed the absence of the liver segment in the IVC and compensatory enlargement of the azygos vein (Figure 3). The hepatic venous blood flowed directly into the right atrium. The venous blood from the kidney and its inferior portion flowed upward through the azygos vein into the SVC and eventually drained into the right atrium. Moreover, the azygos vein, azygos arch, and SVC experienced compensatory enlargement to enable normal circulation. In addition, we also found other variants in this patient, such as the common hepatic artery originating from the superior mesenteric artery and the natural loss of the pancreatic head.
Finally, the patient received a transvenous implantable dual-chamber pacemaker (A3DR01, Medtronic) through the left axillary vein. The postoperative pacing checks confirmed the functionality of the pacemaker. The follow-up examination 3 months after the surgery indicated that the pacemaker was functioning correctly.
Discussion
The IVC is a large retroperitoneal vein that is usually formed at the level of the fifth lumbar vertebra by the confluence of the right and left common iliac veins. The IVC receives venous blood from the tributaries, including the lumbar, right gonadal, renal, right suprarenal, subphrenic, and hepatic veins (4). Developmental abnormalities of the IVC are relatively rare and often asymptomatic. Normal IVC development occurs between the sixth and eighth weeks of embryonic development (5-8). The major causes of developmental abnormalities in the IVC include abnormal continuation or degeneration of veins during embryonic development and acquired intrauterine or perinatal venous thrombosis (5). The patient in this report had an AC of the IVC that probably occurred due to the failure of right vitelline-subcardinal anastomosis to form during embryonic development (9). The failure of the IVC renal segment to anastomose with the hepatic vein resulted in blood flow from the IVC renal segment into the SVC via the enlarged azygos system (Figure 4).
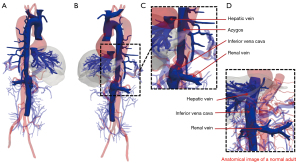
In most cases, AC of the IVC does not affect the functionality of the circulatory system. However, in surgical interventions, such as transthoracic esophagectomy, the dissection of an abnormal IVC might result in a large venous hemorrhage with fatal consequences (10). Preoperative CT venography can reliably identify abnormally developed IVCs that cannot be observed in a physical examination and laboratory tests.
Pacemakers have been widely used in treating severe cardiac arrhythmias alongside other methods, including catheter ablation and medication. In traditional pacemaker placement, the leads are placed through veins to the heart, and a generator is surgically implanted in the body. This approach is still the mainstream option despite the risk of numerous complications, most of which are lead-related, such as hematoma, pocket infection, pneumothorax, cardiac perforation, and lead dislodgement (11). A novel technique is fixing the electrical disconnect with a Micra LP, which is a device that can be implanted in the right ventricle. LP implantation is a viable alternative to single-ventricle pacemakers. An LP could reduce the risks of many lead- and pocket-related complications induced by transvenous implantation of pacemakers (12,13). The primary indications of LP implantation include chronic atrial fibrillation with an AV block or significant pauses, sinus rhythm with a high-grade AV block and a low level of physical activity, sinus bradycardia with infrequent pauses, and unexplained syncope with abnormal electrophysiological findings, such as a prolonged His bundle-ventricular interval (14). Usually, the LP is chosen due to conditions that preclude the implantation of transvenous pacemakers, such as compromised venous access, tricuspid insufficiency, thrombosis, a history of infection, the need to preserve veins for hemodialysis, the expectation that pacing would not be frequent, patients of advanced age, and the patient preference for new technology (13). The LP is often implanted through the femoral vein. Specifically, the delivery catheter is advanced via the femoral vein, iliac vein, and IVC, which are relatively large venous vessels, into the right atrium. It is deflected by pulling the deflection button on the catheter handle so the LP can enter the right ventricle (15). Only one case of AC of the IVC in patients receiving LP implantation has been reported to date (16). In this rare case, the LP was implanted successfully due to the presence of a large primum atrial septal defect and an inlet ventricular septal defect. However, those findings do not apply to other cases of AC of the IVC because of the extremely low probability of occurrence.
The implantable artificial LP was only approved for the Chinese market in November 2019. With the limited clinical practice of LP implantation, there are a lack of clinical guidelines regarding the preoperative assessment of venous anomalies. CT venography has not been commonly used as a screening tool prior to implantation. We believe that the procedure should be reconsidered in similar cases. Therefore, CT venography could be used in some situations to preoperatively assess the patient’s vascular status, avoid intraoperative abandonment, and save ineffective surgical expenses. Some recent studies have reported LP implantation being conducted via the internal jugular vein (17-20). Saleem-Talib et al. (17,18), Kolek et al. (19), and Hale et al. (20) implanted 82, 1, and 1 patients, respectively. Their results commonly showed that the jugular approach seemed to be as safe as the femoral approach. Therefore, the jugular approach could be considered an alternative implantation method for an LP. However, the jugular approach has not been commonly adopted, which might be because a large learning curve exists (18). Despite the limited experience of current surgeons in China with this method, the internal jugular approach deserves further investigation. It could be considered in patients for whom the femoral approach is impossible or undesirable.
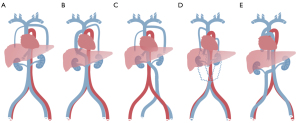
To ensure safe and effective LP implantation, cardiologists must understand the cardiac and venous anomalies that can affect LP implantation, such as congenital heart disease, severe stenosis of the veins, and venous thrombosis. In addition to AC, some other IVC variants can affect LP implantation, mainly because of the discontinuity or high curvature of the IVC (Figure 5). Some typical variants are left-sided IVC, interruption of the infrarenal section of the IVC, and the coexistence of multiple anomalies. First, left-sided IVC has a prevalence of approximately 0.2–0.5% (21). The left IVC joins the left renal vein, which subsequently forms the right renal vein in a normal manner across the ventral side, and the suprarenal segment of the IVC is normal. Second, during interruption of the infrarenal IVC of the IVC, the suprarenal segment is normal, and blood from the lower half part of the body reaches the heart through the azygos–hemiazygos system via SVC. The confluence of the renal veins forms the normal suprarenal vena cava. Third, a variant can be caused by the coexistence of multiple anomalies, such as a double IVC with a retro-aortic right renal vein, interruption of the hepatic segment, and hemizygote continuation of the IVC or double IVC with retro-aortic left renal vein and azygous continuation of IVC (9,22). The relevant sites are anatomically complex, and there are limitations to state-of-the-art radiological imaging techniques, such as the high cost of SVC venography with DSA, low accuracy of magnetic resonance imaging (MRI), or inability to show veins. Therefore, the ECG-gated CT venography can be considered a prescreening tool to reliably detect the anatomical abnormalities preoperatively and avoid the risk of a second operation. The jugular approach provides an alternative option when unexpected anatomical abnormalities are found.
Conclusions
This case report raises awareness for cardiologists about the possibility of IVC disruption that may cause the IVC to not enter directly into the right atrium. As a result, LP implantation cannot be successfully performed through the femoral vein, and the LP may even enter the wrong location. Furthermore, CT venography can be used to identify IVC-related venous anomalies preoperatively to guide appropriate decision-making.
Acknowledgments
Funding: This study was supported by the National Key Research and Development Program of China (No. 2018YFE0198400) and the National Natural Science Foundation of China (No. 81702958).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-885/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mazzucco A, Bortolotti U, Stellin G, Gallucci V. Anomalies of the systemic venous return: a review. J Card Surg 1990;5:122-33. [Crossref] [PubMed]
- Bass JE, Redwine MD, Kramer LA, Huynh PT, Harris JH Jr. Spectrum of congenital anomalies of the inferior vena cava: cross-sectional imaging findings. Radiographics 2000;20:639-52. [Crossref] [PubMed]
- Darlington D, Brown P, Carvalho V, Bourne H, Mayer J, Jones N, Walker V, Siddiqui S, Patwala A, Kwok CS. Efficacy and safety of leadless pacemaker: A systematic review, pooled analysis and meta-analysis. Indian Pacing Electrophysiol J 2022;22:77-86. [Crossref] [PubMed]
- Bubb K, du Plessis M, Hage R, Tubbs RS, Loukas M. The internal anatomy of the inferior vena cava with specific emphasis on the entrance of the renal, gonadal and lumbar veins. Surg Radiol Anat 2016;38:107-14. [Crossref] [PubMed]
- Li SJ, Lee J, Hall J, Sutherland TR. The inferior vena cava: anatomical variants and acquired pathologies. Insights Imaging 2021;12:123. [Crossref] [PubMed]
- Ghandour A, Partovi S, Karuppasamy K, Rajiah P. Congenital anomalies of the IVC-embryological perspective and clinical relevance. Cardiovasc Diagn Ther 2016;6:482-92. [Crossref] [PubMed]
- Malaki M, Willis AP, Jones RG. Congenital anomalies of the inferior vena cava. Clin Radiol 2012;67:165-71. [Crossref] [PubMed]
- Smillie RP, Shetty M, Boyer AC, Madrazo B, Jafri SZ. Imaging evaluation of the inferior vena cava. Radiographics 2015;35:578-92. [Crossref] [PubMed]
- Oliveira JD, Martins I. Congenital systemic venous return anomalies to the right atrium review. Insights Imaging 2019;10:115. [Crossref] [PubMed]
- Zhang Y, Ding Z, Mu T, Pan X, Zhang G, Li X. Case Report: Esophagectomy and Azygos Continuation of the Inferior Vena Cava: A Lethal Combination. Front Cardiovasc Med 2022;9:780646. [Crossref] [PubMed]
- Mond HG, Proclemer A. The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators: calendar year 2009--a World Society of Arrhythmia's project. Pacing Clin Electrophysiol 2011;34:1013-27. [Crossref] [PubMed]
- Ritter P, Duray GZ, Zhang S, Narasimhan C, Soejima K, Omar R, Laager V, Stromberg K, Williams E, Reynolds D. The rationale and design of the Micra Transcatheter Pacing Study: safety and efficacy of a novel miniaturized pacemaker. Europace 2015;17:807-13. [Crossref] [PubMed]
- Reynolds D, Duray GZ, Omar R, Soejima K, Neuzil P, Zhang S, et al. A Leadless Intracardiac Transcatheter Pacing System. N Engl J Med 2016;374:533-41. [Crossref] [PubMed]
- Lee JZ, Mulpuru SK, Shen WK. Leadless pacemaker: Performance and complications. Trends Cardiovasc Med 2018;28:130-41. [Crossref] [PubMed]
- Okabe T, Afzal MR, Houmsse M, Makary MS, Elliot ED, Daoud EG, Augostini RS, Hummel JD. Tine-Based Leadless Pacemaker: Strategies for Safe Implantation in Unconventional Clinical Scenarios. JACC Clin Electrophysiol 2020;6:1318-31. [Crossref] [PubMed]
- Ezhumalai B, Singh Makkar J. Transcatheter leadless permanent pacemaker in complex congenital heart disease with interrupted inferior vena cava: A challenging implantation. Indian Pacing Electrophysiol J 2022;22:165-8. [Crossref] [PubMed]
- Saleem-Talib S, van Driel VJ, Chaldoupi SM, Nikolic T, van Wessel H, Borleffs CJW, Ramanna H. Leadless pacing: Going for the jugular. Pacing Clin Electrophysiol 2019;42:395-9. [Crossref] [PubMed]
- Saleem-Talib S, van Driel VJ, Nikolic T, van Wessel H, Louman H, Borleffs CJW, van der Heijden J, Cox M, Ramanna H. The jugular approach for leadless pacing: A novel and safe alternative. Pacing Clin Electrophysiol 2022;45:1248-54. [Crossref] [PubMed]
- Kolek MJ, Crossley GH, Ellis CR. Implantation of a MICRA Leadless Pacemaker Via Right Internal Jugular Vein. JACC Clin Electrophysiol 2018;4:420-1. [Crossref] [PubMed]
- Hale BW, Bradley DJ, Zampi JD, Whiteside W, Cunnane R. First-in-human combined transcatheter tricuspid valve implantation with leadless VDD pacemaker via left internal jugular approach. HeartRhythm Case Rep 2021;8:155-9. [Crossref] [PubMed]
- Demos TC, Posniak HV, Pierce KL, Olson MC, Muscato M. Venous anomalies of the thorax. AJR Am J Roentgenol 2004;182:1139-50. [Crossref] [PubMed]
- Verma M, Pandey NN, Ojha V, Kumar S, Ramakrishnan S. Developmental Anomalies of the Inferior Vena Cava and its Tributaries: What the Radiologist Needs to Know? Indian J Radiol Imaging 2022;32:355-64. [Crossref] [PubMed]


