
Carotid-cavernous sinus fistula with primary clinical manifestation of cerebral infarction: description of two cases
Introduction
Based on the nomenclature of Barrow, carotid-cavernous fistula (CCF) can be categorized into 4 types (types A–D) (1). Type A CCFs are direct, high-flow lesions connecting the internal carotid artery (ICA) directly to the cavernous sinus. Type A CCFs often result from a single tear in the carotid artery wall, caused either by trauma or aneurysm rupture. These are by far the most common type of CCFs, accounting for approximately 75–80% of CCFs overall. Types B, C, and D CCFs are all indirect, low-flow lesions that arise from meningeal branches of either the ICA or external carotid artery. Type B CCFs arise from meningeal branches of the ICA, type C CCFs arise from meningeal branches of the external carotid artery, and type D CCFs arise from meningeal branches of the ICA or external carotid artery. Posttraumatic CCFs are the most common type, accounting for up to 75% of all CCFs. They have been reported to occur in 0.2% of patients with craniocerebral trauma and in up to 4% of patients who sustain a basilar skull fracture (2). Approximately 80% of patients with CCF have ocular symptoms (3-6). We report 2 rare cases of CCF without ocular symptoms, mainly manifesting as cerebral infarction.
Case presentation
Case 1
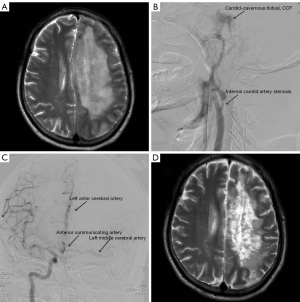
A 64-year-old male was transferred from another hospital with a 5-day headache and 2-day right limb hemiparesis following a traffic accident. On admission, the patient had hemiparesis of the left limb and aphasia. Physical examination revealed the following: clear consciousness, aphasia, free movement of both eyes, no conjunctival congestion, no pulsating exophthalmos, no intracranial vascular murmurs, grade I muscular power of the right limb, and grade V muscular power of the left limb. Magnetic resonance imaging (MRI) of head showed left frontal and parietal massive cerebral infarction (Figure 1A). Cerebrovascular digital subtraction angiography (DSA) suggested moderate stenosis of the left ICA, CCF of the left ICA (Figure 1B), and no blood flow in the distal left ICA; the left middle cerebral artery and the anterior cerebral artery were supplied by the anterior communicating artery (Figure 1C). The patient’s CCF had low blood flow, and it could adequately return through the draining veins of the cavernous sinus, so there was no clinical manifestation of increased cavernous sinus pressure. The patient had no ocular symptoms, such as pulsating exophthalmos or conjunctival congestion. His family members opted for nonsurgical treatment due to concerns about ischemia-reperfusion injury to the left cerebral hemisphere after fistula of the carotid-cavernous was sealed. He was treated with nimodipine, monosialotetrahexosylganglioside (GM1), limb function training, and hyperbaric oxygen therapy, and the muscle power of the right limb recovered to grade III after 3 months. Head MRI showed that the cerebral infarction had improved (Figure 1D). Rehabilitation treatment was continued at a later stage.
Case 2
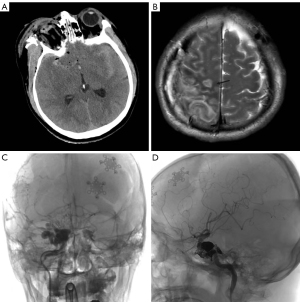
The patient, a 45-year-old male, was admitted to the emergency room with a head and face injury caused by fireworks. Physical examination on admission revealed the following: mental coma, ruptured right eye, and grade 1 muscle power of the left limb. Computed tomography (CT) of the head showed a right frontotemporal parietal epidural hematoma, a right frontal cerebral contusion, a right orbital fracture, a skull base fracture, multiple facial fractures, and a small left frontal epidural hematoma and rupture of the right eyeball (Figure 2A). After admission, an emergency craniotomy was performed to remove the right frontotemporal parietal epidural hematoma plus decompression of the debridement flap plus removal of the right eye. A repeat head CT scan on the next day showed an enlarged left frontal epidural hematoma up to 30 mL. A craniotomy of the left frontal epidural hematoma was performed under general anesthesia. After the operation, the patient’s clear consciousness gradually returned, and the muscle power of the left limb recovered to grade IV. Sixteen days later, a head MRI scan showed right parietal cerebral infarction (Figure 2B). Cerebrovascular DSA showed a right CCF 19 days later (Figure 2C). Since the patient’s right eye had been removed, he had no ocular symptoms. The main clinical manifestation of the patient’s CCF was right parietal cerebral infarction. Endovascular embolization was performed for the treatment of the right CCF one-and-a-half months later. During the operation, 7 coils and 3 mL of Onyx (Medtronic, Inc. Minneapolis, Minnesota, USA) were used to completely embolize the fistula (Figure 2D). One month after endovascular treatment, the muscle power of the patient’s left limb had recovered to grade V.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). With adequate communication, written informed consent was provided by the patients or their family members for publication of the case presentation and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
CCF is a disease in which the arterial wall of the carotid-cavernous sinus segment is damaged, resulting in abnormal arteriovenous communication. The ICA and its meningeal branches cross the cavernous sinus. Once these arteries rupture, the arterial blood flows directly into the venous sinus, causing the sinus pressure to rise and reverse flow into the superior ophthalmic vein, which restricts blood flow back to the eye. This induces ocular symptoms such as pulsatile exophthalmos, ocular motility disorders, bulbar conjunctival congestion, and progressive vision loss (1-4). Many patients are first admitted to ophthalmology departments for obvious eye discomfort, when in fact it is a neurosurgical condition. In a very small number of patients, cerebral ischemia or even cerebral infarction may occur due to excessive flow of the fistula or poor collateral circulation, as well as blood leakage from the fistula of the CCF.
Whole-brain DSA is the gold standard for the diagnosis of CCF, which is also the most important disease assessment method before surgical treatment. During DSA examination, whole-brain DSA including bilateral external carotid arteries should be performed. DSA can clearly show the fistula and the venous drainage pathway behind the fistula. A very small number of patients with mild symptoms and low fistula flow can be cured by compression of the ICA. However, the vast majority of patients with CCF require endovascular treatment to embolize the fistula (5-8). The materials used for embolization are detachable balloons, laminated stents, spring rings, and Onyx (9-12).
We searched the PubMed database with the keyword “carotid-cavernous sinus fistula” and “cerebral infarction”, after which we found only 5 pieces of literature reporting 5 cases of CCF mainly manifested as cerebral infarction; these 5 patients had different causes of cerebral infarction (13-17). Here, we report 2 patients with CCF without ocular symptoms and the main clinical manifestation of cerebral infarction. The first patient had ICA stenosis, so the flow of the fistula was small, and the blood flowing through the fistula could be fully returned through the draining vein, and thus there were no ocular symptoms. The leading causes of cerebral infarction in patients with carotid stenosis are carotid plaque shedding or thrombosis. Cerebral infarction is caused by plaque or thrombus blocking the middle or anterior cerebral artery. The diameter of plaque or thrombus is smaller than the diameter of the ICA. Hence, it will not cause complete blockage of the ICA. DSA of this patient showed that the left anterior cerebral artery and middle cerebral artery were unobstructed. Nevertheless, the distal blood supply of the left ICA was interrupted. Therefore, the cause of cerebral infarction was the interruption of ICA blood supply due to CCF. This is also the world’s first reported case of CCF combined with ICA stenosis and massive cerebral infarction (8,9,13-17). This patient was not treated with endovascular embolization due to concerns about the risk of ischemia–reperfusion injury. Nimodipine, monosialotetrahexosylganglioside, limb function training, and hyperbaric oxygen therapy were administered, and the patient’s cerebral infarction symptoms improved. The second patient also had no ocular symptoms because the patient underwent ophthalmectomy after a traumatic eye rupture. The patient’s left limb was slightly weak, and cerebrovascular DSA indicated that the blood flow of CCF was large, which led to the decrease of the forward blood flow of the right ICA. MRI scans of the head showed focal cerebral infarction in the right parietal lobe. This patient was treated with endovascular embolization for CCF with good results. These 2 cases remind us that patients with head trauma combined with skull base fracture or mandibular fracture, who have symptoms of cerebral ischemia and cerebral infarction need to be considered as having CCF, and cerebrovascular DSA is important for these patients. If CCF is detected, the patient should be treated using an appropriate treatment plan.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-613/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). With adequate communication, written informed consent was provided by the patients or their family members for publication of these cases presentation and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Barrow DL, Spector RH, Braun IF, Landman JA, Tindall SC, Tindall GT. Classification and treatment of spontaneous carotid-cavernous sinus fistulas. J Neurosurg 1985;62:248-56. [Crossref] [PubMed]
- Ellis JA, Goldstein H, Connolly ES Jr, Meyers PM. Carotid-cavernous fistulas. Neurosurg Focus 2012;32:E9. [Crossref] [PubMed]
- Yang L, Tan QQ, Lan CJ, Liao X. Ophthalmic characteristics of carotid cavernous fistula: a case report. Int J Ophthalmol 2021;14:952-4. [Crossref] [PubMed]
- Alam MS, Jain M, Mukherjee B, Sharma T, Halbe S, Jaisankar D, Raman R. Visual impairment in high flow and low flow carotid cavernous fistula. Sci Rep 2019;9:12872. [Crossref] [PubMed]
- Tan AC, Farooqui S, Li X, Tan YL, Cullen J, Lim W, Leng SL, Looi A, Tow S. Ocular manifestations and the clinical course of carotid cavernous sinus fistulas in Asian patients. Orbit 2014;33:45-51. [Crossref] [PubMed]
- Holland LJ, Mitchell Ranzcr K, Harrison JD, Brauchli D, Wong Y, Sullivan TJ. Endovascular treatment of carotid-cavernous sinus fistulas: ophthalmic and visual outcomes. Orbit 2019;38:290-9. [Crossref] [PubMed]
- Ertl L, Brückmann H, Patzig M, Fesl G. Endovascular therapy of direct dural carotid cavernous fistulas - A therapy assessment study including long-term follow-up patient interviews. PLoS One 2019;14:e0223488. [Crossref] [PubMed]
- Khurana M, Alam MS, Balekudaru S, Vijaya L, Madhuri MB, Halbe SV, Noronha VO, George RJ, Mukherjee B. Intraocular Pressure in the Eyes of Patients With Carotid-Cavernous Fistulas: Profile, Intereye Asymmetry, and Treatment Outcomes. J Glaucoma 2019;28:1074-8. [Crossref] [PubMed]
- Shim HS, Kang KJ, Choi HJ, Jeong YJ, Byeon JH. Delayed contralateral traumatic carotid cavernous fistula after craniomaxillofacial fractures. Arch Craniofac Surg 2019;20:44-7. [Crossref] [PubMed]
- Gatto LAM, Tacla R, Koppe GL, Junior ZD. Carotid cavernous fistula after percutaneous balloon compression for trigeminal neuralgia: Endovascular treatment with coils. Surg Neurol Int 2017;8:36. [Crossref] [PubMed]
- Jareczek FJ, Padmanaban V, Church EW, Simon SD, Cockroft KM, Wilkinson DA. Balloon-Assisted Roadmap Technique to Enable Flow Diversion of a High-Flow Direct Carotid-Cavernous Fistula. J Stroke Cerebrovasc Dis 2022;31:106180. [Crossref] [PubMed]
- Kısabay Ak A, Çınar C, Doğan GN, Ataç C, Gökçay F, Çelebisoy N. Clinical improvement in indirect carotid cavernous fistulas treated endovascularly: A patient based review. Clin Neurol Neurosurg 2021;207:106750. [Crossref] [PubMed]
- Ikeda G, Kato N, Watanabe D, Ogata A, Kasuya H, Yamazaki T, Sugita K, Sonobe M. A case of non-traumatic direct carotid-cavernous fistula presenting with cerebral infarction. No Shinkei Geka 2012;40:785-92. [PubMed]
- Iampreechakul P, Tanpun A, Lertbusayanukul P, Siriwimonmas S. Contralateral extensive cerebral hemorrhagic venous infarction caused by retrograde venous reflux into the opposite basal vein of Rosenthal in posttraumatic carotid-cavernous fistula: A case report and literature review. Interv Neuroradiol 2018;24:546-58. [Crossref] [PubMed]
- Wang HX, Bai RL, Huang CG, Lu YC, Zhang GJ. Hemiparesis in carotid cavernous fistulas (CCFs): a case report and review of the literature. Chin J Traumatol 2004;7:317-20. [PubMed]
- Ohshima S, Shigeto H, Kawajiri M, Taniwaki T, Yoshiura K, Kira J. Venous infarction associated with carotid-cavernous fistula. Rinsho Shinkeigaku 2006;46:261-5. [PubMed]
- Kasama A, Katada K, Kanno T, Sugiishi N, Abe M, Shoda M, Ishiyama N, Sano H, Takeshita G. A successful treatment of spontaneous carotid cavernous fistula causing hemorrhagic infarction treated by detachable balloon. No Shinkei Geka 1990;18:631-6. [PubMed]


