
11C-methionine PET/CT and conventional imaging techniques in the diagnosis of primary hyperparathyroidism
Introduction
Primary hyperparathyroidism (PHPT) is caused by a parathyroid adenoma (1). The current way of treating it is parathyroidectomy (PTX). Minimally invasive PTX is preferable and used in case of single and well identified adenoma. In most cases (80% to 90%) only a single parathyroid adenoma leads to the disease, which allows surgeons to operate successfully (1). To be able to perform a minimally invasive PTX, precise preoperative imaging is crucial. There are cases of solitary and multiple adenomas [more specific for multiple endocrine neoplasia (MEN) syndromes], where the imaging may be positive for some glands, but fails to identify all autonomous glands (dual adenomas or multiglandular disease); leading to scheduled bilateral neck exploration or conversion from PTX to bilateral neck exploration during the procedure (2).
Today, several imaging procedures are commonly used in order to detect and localize parathyroid adenomas. Conventional localization techniques include neck ultrasound (US), computed tomography (CT), 99m-Tc-sestamibi scintigraphy. However, the diagnostic potential of the methods mentioned above is limited, mostly in cases of ectopic and multiple adenomas (2).
US is convenient in identifying parathyroid adenoma located on the neck (i.e., close to the thyroid gland or the upper cervical portion of the thymus). However, its diagnostic potential declines a lot in cases of ectopic parathyroid adenomas located behind the trachea, esophagus, or in the mediastinum (3). The sensitivity of US is 76–87% (4). The success of US is highly operator dependent (2). It is worth taking into account that US false positives usually appear to be a thyroid nodule or a lymph node (4).
Mitochondria-rich parathyroid adenoma cells are able to uptake 99m-Tc-sestamibi and scintigraphy is based on this principle (5). It is commonly attributed as the most sensitive and specific imaging modality. In cases of single parathyroid adenoma, sensitivity varies from 80% to 100%. However, 99m-Tc-sestamibi scintigraphy shows relatively poor results in relation to poliglandular PHPT (2). There are several scintigraphy options available (3): washout and subtraction scintigraphy. Scintigraphy may be integrated with single-photon emission computed tomography (SPECT) alone or along with CT.
CT with contrast is a beneficial imaging tool that can help to localize ectopic mediastinal parathyroid glands (6). According to a recent review, the sensitivity of CT is approximately 46–87% (3). Its usage is limited by cost, exposure to radiation, need for iodinated contrast and for an expert radiologist in head & neck imaging (as multiphase CT for parathyroid disorders is not widely available in non-academic centers). It is found that 2-phase CT and 4-phase CT accuracies are not different from each other in parathyroid adenoma localization, but 2-phase CT benefits from having lower radiation exposure (3). 4D CT showed better results than sestamibi SPECT/CT (7).
Lately, great interest is given to the role of positron emission tomography/computed tomography (PET/CT) in PHPT diagnostics (2). It provides information on both anatomical and functional lesion characteristics (8). There were several PET radiotracers implemented in the PHPT assessment. It is described in literature that 11C-methionine may be used for the PHPT diagnostics (9). It retains in the parathyroid adenomas, due to participation in synthesis of the parathyroid hormone (PTH) precursors (1,10). The first reference in literature to 11C-methionine PET/CT in PHPT diagnosis dates to 1994 (11); since the 2010s, its role in the PHPT diagnostics has been underlined. Currently (12-16), 11C-methionine PET/CT is used in cases of prior negative or conflicting adenoma localization results in PHPT patients. It shows a high sensitivity level even in cases of previously failed conventional imaging (8). The role of this method is essential in case of multiple adenomas, which are difficult to locate. 11C-methionine PET/CT is able to become second-line PHPT imaging technique performed after US in centers with nuclear medicine departments with an on-site cyclotron.
Another well-known radiotracer that is used in PHPT diagnostics is 18F-fluorocholine (9). But since our center is equipped with an on-site cyclotron and radiochemical laboratory, we focused our attention on 11C-methionine use, and we did not aim to implement 18F-fluorocholine PET/CT in clinical practice. At our center, 11C-methionine PET/CT is wieldy used in patients with glial tumors.
Thus, the aim of this study was to determine the diagnostic performance of 11C-methionine PET/CT and conventional imaging techniques. We present the following article in accordance with the STARD reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-584/rc).
Methods
Patients
A single-centre retrospective cohort study was conducted. We analyzed the data of 91 patients (age 24–82 years old) diagnosed with PHPT and hospitalized at the Almazov National Medical Research Centre over the past 2 years. Patients formed a consecutive series defined by the eligibility criteria. Medical records, lab results and CT imaging of all patients were studied. Since 2020, PET/CT with 11C-methionine was performed on 45 patients after admission to the hospital. PET/CT results were analyzed in a prospective manner. PHPT was confirmed based on laboratory tests (elevated PTH, high or normal level of serum total or ionized calcium levels) (17). Intact PTH (iPTH, 15.0–65.0 pg/mL) assessment was carried out using immunochemiluminescent assay (Architect i2000SR, Abbott Laboratories and Elycsys 2010, Roche-Diagnostics GmbH), ionized and total calcium (1.11–1.32 and 2.15–2.65 mmol/L, respectively) were measured by autoanalyzer Architect c8000 (Abbott Laboratories). All patients underwent US, 99m-Tc-sestamibi/99m-Tc-pertechnetate subtraction scintigraphy and/or CT. 11C-methionine PET/CT was used as a method in 45 patients. Then, all patients underwent PTX (Figure 1). Histological results were used as a benchmark in order to evaluate the characteristics of imaging modalities.
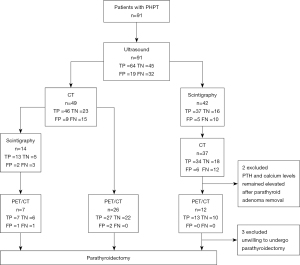
The inclusion criteria were: biochemically confirmed PHPT (elevated iPTH level and hypercalcemia OR twofold elevated iPTH level, and normocalcemia, and 25(OH)D >30 ng/mL, and eGFR >60 mL/min/1.73 m2), indications for PTX (PHPT manifestations, age under 50 years, serum calcium remains >0.25 mmol/L above the upper limit of normal, 24-h calciura >10 mmol/24 h), performed PTX, biochemically cured PHPT after PTX (intraoperative iPTH cut-off values were less than 65.0 pg/mL or 50% decrease from the baseline value and subsequent normocalcemy within the period before being discharged from the hospital). The exclusion criteria were estimated glomerular filtration rate less than 45 mL/min/1.73 m2, acute medical conditions (i.e., myocardial infarction, stroke, etc.), glucocorticosteroids intake, cancer over the past 5 years, alcohol or drug addiction were not included in the study. The patients’ medical records were reviewed to collect medical history including gender, age, preoperative/intraoperative/postoperative iPTH, serum total and ionized calcium, serum phosphorus, 24-hour urine calcium, serum 25(OH)D, and pathology outcome.
Sample size was not pre-calculated, it was composed from the patients who were hospitalized to Almazov National Medical Research Centre and suitable for the eligibility criteria. A time span was set in order to reach the end point of study—biochemically cured PHPT and histologically confirmed parathyroid adenoma or hyperplasia after PTX.
The study was approved by the Ethics Committee of Almazov National Medical Research Center, St. Petersburg, Russia (No. 2004020) and was performed according to the principles of the 1964 Declaration of Helsinki and its later amendments or comparable standards. Written informed consent was obtained from all patients.
Imaging
Neck ultrasonography
The study was carried out on all patients on US system (Vivid 3, GE Healthcare, Marlborough, MA, USA). Supine position with a hyperextended neck using a high-frequency linear transducer was used to perform it. The neck was always examined from the level above the thyroid to the clavicle caudally. US reports were analyzed. Round or oval well-defined, hypoechogenic formation delimited by an echogenic line and surrounded by hyper-echogenic thyroid tissue were interpreted as pathological finding.
99m-Tc-sestamibi/99m-Tc-pertechnetate subtraction scintigraphy
Scintigraphy was performed using 2 radiopharmaceuticals (99m-Tc-sestamibi (MIBI) and 99m-Tc-pertechnetate) and a dual heads gamma camera system (E.CAM, Siemens Medical Solutions, Erlangen, Germany). Patients underwent planar neck scintigraphy. Initially scans were obtained after administration of pertechnetate 70 MBq. On the next day, the early phase of planar MIBI scintigraphy was performed 10 minutes after intravenous injection of 700 MBq MIBI, and SPECT in early phase was followed to planar scans. Planar and SPECT delayed scans were performed 2 hours after MIBI administration. Subtraction scintigraphy was carried out using gamma camera software. The pertechnetate scan was subtracted from the early and delayed MIBI scan. The areas of increased MIBI uptake at the delayed and the subtraction images were considered to be a positive result. Reports were analyzed.
CT
The study was performed using a 128-row multidetector CT-scanner (Philips Ingenuity CT, Cleveland, USA). Iodinated contrast material (300 mg I/mL concentration, 100 mL at 4.5 mL/second) was infused through an 18-gauge cannula placed in the right cubital vein and followed by a 40-mL saline chaser. Unenhanced and double contrast phase images were obtained from the level of the mandible to the level of the carina. Arterial and delayed phases were performed at 25 s and 80 s after the start of the contrast injection. Bolus tracking was used to optimize dynamic contrast enhancement. The following parameters were used for all 3 phases: 0.625-mm section thickness; tube rotation time 0.75 second; pitch factor 1.014; 140 kVp; Dose right index 33, and automatic tube current modulation. 1 mm thick contiguous axial images were reconstructed using iterative reconstruction algorithm (IMR, Philips).
Images were analyzed separately by a radiologist with 10 years of experience in neck and head CT, examining about 100 imagings of this kind annually. Software (Philips IntelliSpace, version 6.0, for the Philips Medical Systems scanner), was used for CT review. It allowed us to scroll through the CT images in the axial, coronal and sagittal planes and reconstructing 3D images. Lesions’ localization, size, CT-density (in nonenhanced phase, in the arterial phase and washing out, in the venous phase) were evaluated based on CT-scan information. Enhancement types were (18,19): (A) greater in attenuation than thyroid on arterial phase (hyperenhance), can have any appearance on delayed phase; (B) similar or lower in attenuation than thyroid on arterial phase, lower in attenuation (washout) on delayed phase; (C) neither hyperenhance on arterial phase nor washout on delayed phase. Additional findings, like an enlarged polar vessel or cystic degeneration were also evaluated.
The interpretation scoring system was as follows (19): consistent with—type A or B enhancement plus at least one additional finding; suspicious for—type A or B enhancement without an additional finding or type C enhancement plus at least one additional finding; possible—type C enhancement without an additional finding.
All of above types were interpreted as pathological hyperfunctioning parathyroid tissue (positive). Round- or oval-shaped lesions with soft tissue CT-density and progressive enhancement for approximately 90 seconds, corresponding to the delayed phase) were interpreted as lymphatic nodes (negative).
11C-methionine PET/CT
We performed PET/CT using scanner Discovery 710 (GE Healthcare, Milwaukee, WI, USA). Static scans were performed 10 min after intravenous injection of 350–600 MBq 11C-methionine. PET/CT scans were acquired from the base of the skull to the diaphragm to capture possible ectopic adenomas. Low-dose CT transmission data was initially obtained. Then PET static scans were carried out, each taking 10 minutes.
After that CT was performed with contrast media in native, arterial, venous, and delayed phases. Delayed scans were performed 80 seconds after contrast injection. CT was evaluated according to the scheme mentioned before. PET features of parathyroid adenoma (positive result) were a focus of high 11C-methionine uptake with or without typical anatomical abnormalities according to CT-data. The obtained images were analyzed separately by a radiologist with 20 years of experience in nuclear medicine, examining on average 100 imagings of this kind annually.
The possible sources of variability in test accuracy are the experience of readers, the setting and previous test results. We tried to minimize the impact of these factors within this study. US, CT, scintigraphy, and PET/CT results were labeled separately. An experienced radiologist reviewed CT and PET/CT imaging—one expert for each method. Another person analyzed the histology results and determined a true or false outcome. Nevertheless, the listed aspects may affect the final results.
Surgery and histology
All the PTXs were performed by the same 3 experienced surgeons in Almazov National Medical Research Center. In cases of single adenoma, the surgical approach included minimally invasive PTX with subsequent intraoperative iPTH assessment. Difficulties in adenoma localization or the presence of 2 enlarged ipsilateral glands, have led to conversion into bilateral cervical exploration.
Surgery was considered successful after an intraoperative iPTH serum level decrease of more than 50% from the baseline value or into the normal range (2).
The size of all removed parathyroid glands were measured. All removed samples underwent histological examination, the results were used as the benchmark in order to evaluate the diagnostic accuracy of the studied methods. Successful imaging was observed in cases with the detected lesion identified and removed via surgery, and confirmed as an adenoma or hyperplasia and the patient was biochemically cured (intraoperative iPTH cut-off values were less than 65.0 pg/mL or 50% decrease from the baseline value and subsequent normocalcemy within the period before discharging from the hospital). The pathology diagnosis was based on WHO Classification of Tumors of Endocrine Organs [2017]. Adenomas were characterized by well circumscribed, frequently with thin fibrous capsule, absent or reduced stromal adipocytes, compressed nonneoplastic, most commonly composed of chief cells, mitoses and bizarre nuclei (endocrine atypia) may be focally present. Atypical adenomas had borderline features concerning for malignancy. Histological presentation corresponded to dense fibrous bands with hemosiderin, prominent nuclear atypia with spindled nuclei, notable mitotic activity, adherence to adjacent tissue, necrosis, solid or trabecular growth. But there was no evidence of lymphovascular invasion, perineural invasion, and invasion into adjacent structures or metastasis in cases of atypical adenomas. Findings that were not histologically verified as an adenoma or hyperplasia were considered as false positive or true negative depending on the imagining result. Findings that were histologically verified as an adenoma or hyperplasia were considered as false negatives when the imagining result did not show the parathyroid lesion. Based on this, the CT, PET/CT images were reviewed and labeled as true positive or negative and false positive or negative. US and 99m-Tc-sestamibi/99m-Tc-pertechnetate subtraction scintigraphy medical records were analyzed and labeled the same way. The following formulas were used in order to estimate measures of diagnostic accuracy (sensitivity and estimated specificity). The number of true positives divided by the sum of true positives and false negatives resulted into sensitivity. The number of true negatives divided by the sum of true negatives and false positives resulted into specificity. The possible sources of variability in test accuracy are the experience of readers, the setting and previous test results. We tried to minimize the impact of these factors within this study. First US, CT, scintigraphy, and PET/CT results were labeled separately. An experienced radiologist reviewed CT and PET/CT imaging—one expert for each method. Another person analyzed the histology results and concluded whether outcomes were true or false. But nevertheless, the listed aspects may affect the final results. STATISTICA 10.0 software (STATISTICA (RRID:SCR_014213) was used in statistical analysis). All variables demonstrated non-normal distribution, hence, non-parametric criteria were implemented. Continuous variables were characterized by medians (Me) and quartiles (25 to 75). The Mann-Whitney U-test was used for quantitative characteristics’ pairwise comparisons. The comparision between discrete characteristics was performed with the Chi-square test (Fisher’s exact test was used for binary characteristics). P values <0.05 were considered statistically significant.
Results
91 patients (female: male ratio of 11:1; median age 59 years) were included in this study. The majority of PHPT patients were women (88%), median age was 59 (51 to 68) years. PHPT patients’ laboratory and demographic characteristics are presented in Table 1.
Table 1
| Variables | Median | Lower quartile | Upper quartile |
|---|---|---|---|
| Age (years) | 58.7 | 51.0 | 68.0 |
| Weight (kg) | 73.5 | 62.0 | 84.0 |
| Height (cm) | 163.3 | 159.0 | 166.0 |
| BMI (kg/m2) | 27 | 25 | 30 |
| Preoperative serum iCa (mmol/L) | 1.5 | 1.4 | 1.6 |
| Preoperative serum total Ca (mmol/L) | 3.1 | 2.8 | 3.1 |
| 24-h calciura (mmol/24 h) | 7.1 | 4.2 | 9. 0 |
| Serum P (mmol/L) | 0.9 | 0.8 | 1.1 |
| Preoperative iPTH (pmol/L) | 237.9 | 105.3 | 264.2 |
| 25(OH)D (ng/mL) | 29.4 | 16.4 | 37.2 |
| Postoperative serum iCa (mmol/L) | 1.3 | 1.2 | 1.4 |
| Postoperative serum total Ca (mmol/L) | 2.4 | 2.3 | 2.4 |
| Intraoperative iPTH (pg/mL) | 28.0 | 10.3 | 38.0 |
| eGFR† (mL/min/1.73 m2) | 83.1 | 65.0 | 100.0 |
| T-score L1–L4 (SD) | −1.5 | −2.7 | −0.3 |
| T-neck (SD) | −1.9 | −2.5 | −1.3 |
| T-score 33% radius (SD) | −2.4 | −3.3 | −1.7 |
†, calculated by CKD-EPI formula. PHPT, primary hyperparathyroidism; BMI, body mass index; iCa, ionized calcium; total Ca, total calcium; 24-h calciura, 24-hour urine calcium; P, phosphorus; iPTH, intact PTH; 25(OH)D, 25-hydroxyvitamin D; eGFR, estimated glomerular filtration rate.
Clinical features of PHPT included bone (fragility fractures, osteitis fibrosa cystic), kidney (nephrolithiasis, nephrocalcinosis) and gastrointestinal (erosive gastritis, erosive reflux esophagitis peptic ulcer disease, and gallstones) manifestations. Ectopic adenomas were characterized by significantly higher (P=0.003) serum calcium level (3.09 mmol/L) rather than typically located ones (2.85 mmol/L). All patients underwent PTX and were biochemically cured after it. The following data on lesions was obtained. Ninety-eight lesions were removed in total. The vast majority were represented by a single adenoma (84 adenomas; 86%), 19 adenomas were ectopic. Multiple (double) adenomas were identified in 2 cases. Hyperplasia of 2 parathyroid glands was found in 5 patients. The median volume of ectopic adenomas was significantly higher than the volume of non-ectopic (2.45 and 0.54 cm3, respectively, P<0.001). The median volume of multiple lesions was significantly lower than the volume of single ones (0.24 and 0.77 cm3, respectively, P=0.036).
The numbers of true positives, false positives, true negatives, and false negatives of all methods are presented in Table 2. Diagnostic accuracy of all methods is presented in Table 3.
Table 2
| Examination | TP | FP | TN | FN |
|---|---|---|---|---|
| US | 64 | 19 | 45 | 32 |
| CT | 80 | 15 | 41 | 27 |
| Scintigraphy | 50 | 7 | 21 | 13 |
| PET/CT | 47 | 3 | 38 | 1 |
US, ultrasound; CT, computed tomography; PET/CT, positron emission tomography/computed tomography; TP, true positive; FP, false positive; TN, true negative; FN, false negative.
Table 3
| Accuracy | PET/CT | CT | Scintigraphy | US |
|---|---|---|---|---|
| Sensitivity (%) | 98 | 75 | 79 | 67 |
| Specificity (%) | 93 | 73 | 75 | 70 |
| Diagnostic efficacy (%) | 96 | 74 | 78 | 68 |
| Positive predictive value (%) | 94 | 84 | 88 | 77 |
| Negative predictive value (%) | 97 | 60 | 62 | 58 |
US, ultrasound; CT, computed tomography; PET/CT, positron emission tomography/computed tomography.
US sensitivity and specificity were 67% (95% CI: 56% to 76%) and 70% (95% CI: 57% to 81%), respectively, based on 91-patient US reports. Regarding ectopic localization of lesions, the following results were obtained: the number of false negative US results was significantly higher (P<0.001) among the ectopic lesions (15/19, 79%) rather than in the group of typically located adenomas (17/79, 22%).
CT sensitivity and specificity were 75% (95% CI: 65% to 82%) and 73% (95% CI: 59% to 84%), respectively, which were evaluated across 86 patients. CT volume of US false negative adenomas was significantly higher than the US volume of true positive ones [1.41 (SD =3.12) cm3 and 0.56 (SD =0.42) cm3, respectively, P=0.004].
99m-Tc-sestamibi/99m-Tc-pertechnetate subtraction scintigraphy sensitivity and specificity were 79% (95% CI: 67% to 88%) and 75% (95% CI: 55% to 89%), respectively, based on data of 56-patient reports.
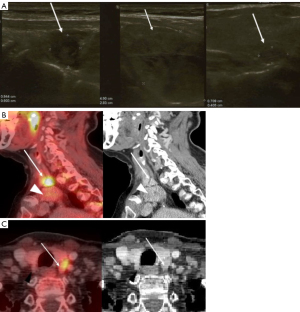
11C-methionine PET/CT was performed on 45 patients. The following results were obtained: sensitivity and specificity of PET/CT was 98% (95% CI: 88% to 100%) and 93% (95% CI: 79% to 98%), respectively. 4 out of 45 patients who underwent PET/CT had multiple lesions that were localized on 11C-methionine PET/CT preoperatively (Figure 2). According to histopathology results, 2 patients had double adenomas and two others—double hyperplasia. Figure 3 shows a case of a positive 11C-methionine PET/CT and discordant US results. 11C-methionine PET/CT showed a lesion located at the back side of the left thyroid gland, suspected to be parathyroid adenoma. US showed 3 lesions. According to the histology result, the lesion on 11C-methionine PET/CT was a parathyroid adenoma.

A group of patients (n=19) with each imaging technique performed were analyzed. Each method’ diagnostic accuracy was evaluated separately within that group. US, 99m-Tc-sestamibi/99m-Tc-pertechnetate subtraction scintigraphy, CT, and 11C-methionine PET/CT demonstrated the following sensitivity rates of 60%, 75%, 75%, and 100% and specificity rates of 62%, 71%, 71%, and 90%, respectively. Today, combinations of techniques are used in PHPT diagnostics and US and scintigraphy are the “gold standard” in clinical practice. Diagnostic accuracy of different methods’ combinations was assessed. US together with scintigraphy or CT had a sensitivity of 80% and 85%, respectively, and a specificity of 90% and 86%, respectively. Scintigraphy along with CT sensitivity was 90% and specificity was 90%. All together, US, scintigraphy, and CT had sensitivity of 90% and 95%, respectively. Additionally, it is worth noting that 11C-methionine PET/CT effective radiation dose is roughly two-times lower than that of both CT and subtraction scintigraphy (14.1, 16, 13.44 mSv, respectively).
No adverse effects during the study were found.
Discussion
11C-methionine PET/CT has a potential to play an important role in the lesion localization presenting anatomical and functional imaging together so this one method may replace both CT and scintigraphy in patients with PHPT. It allows localizing a lesion within only one procedure.
This study evaluates the diagnostic accuracy of 11C-methionine PET/CT, US, CT, and 99m-Tc-sestamibi/99m-Tc-pertechnetate subtraction scintigraphy in patients with PHPT.
There are a few studies evaluating the diagnostic accuracy of 11C-methionine PET/CT.
There is a retrospective study that involved 51 patients (12). These were patients with previous negative or ambiguous conventional imaging including US scanning of the neck (n=15), CT (n=17), magnetic resonance imaging (n=9), venous sampling (n=11), and MIBI scans (n=51). 22 patients were suspected to have PHPT and 29 patients had recurrent/persistent hyperparathyroidism. Of the last ones, 6 had undergone neck explorations in the past, but no abnormal parathyroid glands were found. The diagnosis was confirmed based on histology (n=29) or clinical follow-up (n=22). Found 11C-methionine PET/CT sensitivity was 83%. In a retrospective study of 18 patients, with inconclusive results of US and MIBI-SPECT/CT all included patients underwent 11C-methionine PET/CT and 12 of them received surgery. Per patient sensitivity was 91.7% (11 of 12) and per lesion was 73.3% (11 of 15) (13). There is another retrospective single center cohort study. It included 28 PHPT patients with prior negative MIBI-SPECT/CT and/or US, all of them had PTX. Based on histology results, estimated 11C-methionine PET/CT sensitivity was 72% (1). In another retrospective study of 54 PHPT patients, preoperative localization studies included US (n=54) and 99mTc sestamibi scanning (n=34). If these imaging modalities could not localize the adenoma, then 11C-methionine PET/CT (n=8) was carried out. PTX was performed on 43 patients. The sensitivity of 11C-methionine PET/CT and 99mTc-Sestamibi were 71.4% and 70.6%, respectively (14). The latest similar study involved 38 PHPT patients with inconclusive or doubtful US or MIBI results. Then, both 11C-methionine PET/CT and PTX were performed on all patients. The sensitivity, specificity and accuracy of 11C-methionine PET/CT were established at a level of 79%, 75% and 79%, respectively (15). There are also several studies on 11C-methionine PET/CT diagnostic accuracy that were not focused on PHPT patients with previous inconclusive results from conventional imaging techniques. For example, a study of 16 patients, who all had PTX, was carried out by Chun et al. and it observed a sensitivity of 91.7% (16). There is another prospective study of 14 PHPT patients; in all patients, preoperative MIBI scintigraphy and 11C-methionine PET/CT were performed before PTX. The sensitivity was 100% for MIBI scintigraphy and 76.9% for PET/CT, and the positive predictive value was 85.7% and 90.0%, respectively (20). PET/CT diagnostics accuracy varies from one study to another, but its sensitivity is consistently higher than 70%. In general, the numbers are lower than ones that we have found; this may be due to different study design (PET/CT was often performed after previous inconclusive imaging). Another thing that could have affected the results is that the sample size of patients who underwent PET/CT and then subsequently had PTX is often rather small.
Our results regarding the diagnostic accuracy of 11C-methionine PET/CT are comparable with 18F-fluorocholine PET/CT ones. For instance, there is a prospective study, where the accuracy of 18F-fluorocholine PET/CT was compared with that of 99mTc-MIBI or99mTc-tetrofosmin SPECT/CT in PHPT imaging. The results are based on 82 patients’ analysis. 18F-fluorocholine PET/CT per-lesion sensitivity, specificity, positive predictive value, negative predictive value and overall accuracy were 93.7%, 96.0%, 90.2%, 97.4% and 95.3%, respectively (9). There is a prospective study comparing the diagnostic performance of 18F-fluorocholine PET/CT with 11C-methionine PET/CT. It included 58 patients with PHPT and negative or inconclusive 99mTc-MIBI SPECT, and both 18F-fluorocholine and 11C-methionine PET/CT findings were used for presurgical localization of hyperfunctioning parathyroid tissue. Twenty-six patients were operated on and 21 patients were considered cured after surgery. Per-lesion-based sensitivity and positive predictive value were 84% and 90%, respectively, for 18F-fluorocholine and 52% and 94%, respectively, for11C-methionine (P<0.0001) (21). Thus, this study showed the advantages of 18F-fluorocholine. However, results that we have received are at a higher level.
11C-choline and 68Ga-PSMA are available for prostate cancer diagnostics in our center.
We have episodic experience in 11C-choline’s use in PHPT imaging in our centre. We are unable to make strong conclusions on imaging quality and sensitivity differences in these techniques. However, based on the preliminary results we obtained, it seems that 11C-choline PET imaging quality is higher than that of 11C-methionine, but noticeable sensitivity difference wasn’t witnessed.
It is worth mentioning that the isotope of 11C has a half-life of 20 minutes, which is significantly faster than the half-life of an 18F isotope, at 109 minutes. Thus, the use of radiopharmaceutical with 11C isotope lowers the effective radiation dose from 9 mSv to 3.4–4 mSv.
To our knowledge, there are no prospective studies available where included patients had US, 99m-Tc-sestamibi/99m-Tc-pertechnetate subtraction scintigraphy, CT, 11C-methionine PET/CT, and PTX, that would allow the comparision of imaging techniques with each other.
Found size differences between multiple and single lesions may explain why it’s a challenging problem to find all causative adenomas. Sometimes it is difficult to detect all the lesions after pointing out the first one. In these cases, additional 11C-methionine uptake may play a crucial role in completing the diagnostic search.
Moreover, both subtraction scintigraphy and CT effective radiation dose is 2 times higher than that of 11C-methionine PET/CT. So replacing subtraction scintigraphy and CT with 11C-methionine PET/CT is useful in terms of reducing effective radiation dose. Another well-known PET/CT advantage is that its resolution is higher than in SPECT or in subtraction scintigraphy, so this can lead to higher 11C-methionine PET/CT sensitivity.
A limitation of the study is the small sample size. Further prospective studies are needed in settings where a bigger number of included patients undergoes each imaging technique. Up until then, 11C-methionine PET/CT is going to remain an additional imaging technique. Another thing that will not let 11C-methionine PET/CT become a widely used method is that 11C-methionine has short half-life, leading to the need for a on-site cyclotron.
Conclusions
Our study showed that 11C-methionine PET/CT has higher sensitivity and specificity than conventional techniques and the “gold standard” in PHPT imaging (scintigraphy and US), according to the result obtained on the separate group of 19 patients. These numbers were comparable with the ones gained from the whole sample. Despite the need for an on-site cyclotron, 11C-methionine PET/CT is going to have its own niche in PHPT imaging, in cases where at least the first imaging procedure didn’t indicate an adenoma.
Acknowledgments
The authors would like to express their gratitude to colleagues for helping in patient recruitment.
Funding: This work was financially supported by the Ministry of Science and Higher Education of the Russian Federation (agreement No. 075-15-2022-301).
Footnote
Reporting Checklist: The authors have completed the STARD checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-584/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-584/coif). The authors report that this work was financially supported by the Ministry of Science and Higher Education of the Russian Federation (No. 075-15-2022-301). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Ethics Committee of Almazov National Medical Research Center, St. Petersburg, Russia (No. 2004020) and was performed according to the principles of the 1964 Declaration of Helsinki and its later amendments or comparable standards. Written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Noltes ME, Coester AM, van der Horst-Schrivers ANA, Dorgelo B, Jansen L, Noordzij W, Lemstra C, Brouwers AH, Kruijff S. Localization of parathyroid adenomas using (11)C-methionine pet after prior inconclusive imaging. Langenbecks Arch Surg 2017;402:1109-17. [Crossref] [PubMed]
- Walker MD, Bilezikian JP. Primary Hyperparathyroidism. Endotext [Internet]. South Dartmouth (MA); 2021 [updated 2021 Apr 19, cited 2022 Mar 28]. Available online: https://www.ncbi.nlm.nih.gov/books/NBK278923/
- Khan AA, Hanley DA, Rizzoli R, Bollerslev J, Young JE, Rejnmark L, et al. Primary hyperparathyroidism: review and recommendations on evaluation, diagnosis, and management. A Canadian and international consensus. Osteoporos Int 2017;28:1-19. [Crossref] [PubMed]
- Cheung K, Wang TS, Farrokhyar F, Roman SA, Sosa JA. A meta-analysis of preoperative localization techniques for patients with primary hyperparathyroidism. Ann Surg Oncol 2012;19:577-83. [Crossref] [PubMed]
- Kunstman JW, Kirsch JD, Mahajan A, Udelsman R. Clinical review: Parathyroid localization and implications for clinical management. J Clin Endocrinol Metab 2013;98:902-12. [Crossref] [PubMed]
- Noureldine SI, Aygun N, Walden MJ, Hassoon A, Gujar SK, Tufano RP. Multiphase computed tomography for localization of parathyroid disease in patients with primary hyperparathyroidism: How many phases do we really need? Surgery 2014;156:1300-6; discussion 13006-7. [Crossref] [PubMed]
- Yeh R, Tay YD, Tabacco G, Dercle L, Kuo JH, Bandeira L, McManus C, Leung DK, Lee JA, Bilezikian JP. Diagnostic Performance of 4D CT and Sestamibi SPECT/CT in Localizing Parathyroid Adenomas in Primary Hyperparathyroidism. Radiology 2019;291:469-76. [Crossref] [PubMed]
- Tang BN, Moreno-Reyes R, Blocklet D, Corvilain B, Cappello M, Delpierre I, Devuyst F, Van Simaeys G, Goldman S. Accurate pre-operative localization of pathological parathyroid glands using 11C-methionine PET/CT. Contrast Media Mol Imaging 2008;3:157-63. [Crossref] [PubMed]
- Beheshti M, Hehenwarter L, Paymani Z, Rendl G, Imamovic L, Rettenbacher R, Tsybrovskyy O, Langsteger W, Pirich C. (18)F-Fluorocholine PET/CT in the assessment of primary hyperparathyroidism compared with (99m)Tc-MIBI or (99m)Tc-tetrofosmin SPECT/CT: a prospective dual-centre study in 100 patients. Eur J Nucl Med Mol Imaging 2018;45:1762-71. [Crossref] [PubMed]
- Habener JF, Maunus R, Dee PC, Potts JT Jr. Early events in the cellular formation of proparathyroid hormone. J Cell Biol 1980;85:292-8. [Crossref] [PubMed]
- Hellman P, Ahlström H, Bergström M, Sundin A, Långström B, Westerberg G, Juhlin C, Akerström G, Rastad J. Positron emission tomography with 11C-methionine in hyperparathyroidism. Surgery 1994;116:974-81. [PubMed]
- Beggs AD, Hain SF. Localization of parathyroid adenomas using 11C-methionine positron emission tomography. Nucl Med Commun 2005;26:133-6. [Crossref] [PubMed]
- Braeuning U, Pfannenberg C, Gallwitz B, Teichmann R, Mueller M, Dittmann H, Reimold M, Bares R. 11C-methionine PET/CT after inconclusive 99mTc-MIBI-SPECT/CT for localisation of parathyroid adenomas in primary hyperparathyroidism. Nuklearmedizin 2015;54:26-30. [Crossref] [PubMed]
- Mallikarjuna VJ, Mathew V, Ayyar V, Bantwal G, Ganesh V, George B, Hemanth GN, Vinotha P. Five-year Retrospective Study on Primary Hyperparathyroidism in South India: Emerging Roles of Minimally Invasive Parathyroidectomy and Preoperative Localization with Methionine Positron Emission Tomography-Computed Tomography Scan. Indian J Endocrinol Metab 2018;22:355-61. [Crossref] [PubMed]
- Maccora D, Caldarella C, Calcagni ML. (11)C-Methionine PET/CT in patients with primary hyperparathyroidism and inconclusive pre-operative imaging work-up: diagnostic accuracy and role of semi-quantitative analysis. Ann Nucl Med 2021;35:469-78. [Crossref] [PubMed]
- Chun IK, Cheon GJ, Paeng JC, Kang KW, Chung JK, Lee DS. Detection and Characterization of Parathyroid Adenoma/Hyperplasia for Preoperative Localization: Comparison Between (11)C-Methionine PET/CT and (99m)Tc-Sestamibi Scintigraphy. Nucl Med Mol Imaging 2013;47:166-72. [Crossref] [PubMed]
- Mokrysheva NG, Eremkina AK, Mirnaya SS, Krupinova JA, Voronkova IA, Kim IV, et al. The clinical practice guidelines for primary hyperparathyroidism, short version. Probl Endokrinol (Mosk) 2021;67:94-124. [Crossref] [PubMed]
- Hoang JK, Sung WK, Bahl M, Phillips CD. How to perform parathyroid 4D CT: tips and traps for technique and interpretation. Radiology 2014;270:15-24. [Crossref] [PubMed]
- Kuzminski SJ, Sosa JA, Hoang JK. Update in Parathyroid Imaging. Magn Reson Imaging Clin N Am 2018;26:151-66. [Crossref] [PubMed]
- Martínez-Rodríguez I, Martínez-Amador N, de Arcocha-Torres M, Quirce R, Ortega-Nava F, Ibáñez-Bravo S, Lavado-Pérez C, Bravo-Ferrer Z, Carril JM. Comparison of 99mTc-sestamibi and 11C-methionine PET/CT in the localization of parathyroid adenomas in primary hyperparathyroidism. Rev Esp Med Nucl Imagen Mol 2014;33:93-8. [Crossref] [PubMed]
- Mathey C, Keyzer C, Blocklet D, Van Simaeys G, Trotta N, Lacroix S, Corvilain B, Goldman S, Moreno-Reyes R. (18)F-Fluorocholine PET/CT Is More Sensitive Than (11)C-Methionine PET/CT for the Localization of Hyperfunctioning Parathyroid Tissue in Primary Hyperparathyroidism. J Nucl Med 2022;63:785-91. [PubMed]


