
Prolonged imaging time in the salivagram appears unnecessary for detecting aspiration
Introduction
Aspiration, the inhalation of oropharyngeal or gastric contents into the lower respiratory tract and larynx (1), is an important reason for morbidity and mortality from respiratory disease in children (2). Recurrent lung infections can be caused by aspiration, so early diagnosis and treatment are important to prevent lung damage (3). Barium swallow, gastroesophageal reflux scan (milk scan), and salivagram have been used for the diagnosis of aspiration (4). However, performing a barium swallow in some pediatric patients, particularly neonates, is frequently impractical. In addition to aspiration, a gastroesophageal reflux scan can also be used for the diagnosis of gastroesophageal reflux. The salivagram was first described many decades ago by the physicians at The Children’s Hospital of Philadelphia to detect possible lung aspiration in infants or young children (5). Yang et al. compared the diagnostic value of a gastroesophageal reflux scintigraphy and a radionuclide salivagram in the detection of pulmonary aspiration in pediatric patients. In patients who underwent both examinations, the detection rate was 1.9% for gastroesophageal reflux scintigraphy and 22.2% for radionuclide salivagram, and radionuclide salivagram had a much higher detection rate for pulmonary aspiration than gastroesophageal reflux scintigraphy (6). In addition, Shao et al. introduced a semi-quantitative analytical method to optimize and complement the current classification of pulmonary aspiration detected by salivagram (7).
The original protocol for a salivagram was acquired within 60 minutes, which has high sensitivity. However, in select cases, it is difficult or impractical to have a 60-minute image acquisition. Society’s nuclear medicine and molecular imaging guidelines recommend 30–60-minute dynamic imaging, and some institutions therefore only acquire 30-minute images. However, there is insufficient data to show whether shortened imaging times are adequate to detect aspiration. The retrospective study aims to determine whether a shorter period of image acquisition can be adopted without significantly lowering the test’s sensitivity for detecting aspiration.
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Institutional Review Board of Beijing Friendship Hospital, Capital Medical University approved the study, and the requirement for written informed consent was waived due to the retrospective nature of the study.
A total of 398 positive salivagram patients (ages 1 month to 9 years old, average age was 5.2 months; median age was 4.5 months) were included in the study from January 2014 to December 2018 in our hospital. All these patients performed the salivagram due to suspicion of pulmonary aspiration associated with a cough and a recurrent respiratory infection. The exclusion criteria included: (I) patients with a nasogastric tube or gastric tube for feeding; (II) premature babies with gestational ages less than 37 weeks; (III) babies with birthweights under 2,500 g; and (IV) patients with neurological diseases.
Salivagram
The protocol of salivagrams used in this study has been reported previously (6). The 99mTc sulfur colloid was diluted into 1 mL of normal saline and administered on the tongue. The dose of 99mTc sulfur colloid was calculated based on its weight of 0.55 MBq/kg. The dose range was from 7.4 MBq to 37 MBq (about 0.05 mSv of total body effective dose for every patient). During the scan, the children may be awake or asleep. The images from the mouth to the upper abdomen were acquired within 60 minutes (low-energy, high-resolution collimator). After the dynamic images, posterior static images of the thorax and upper abdomen were similarly acquired to evaluate pulmonary aspiration. During reading these images, potential contamination of the neck, thorax, or clothes should be taken care of. Activity in the area of the lung field, bronchi, or tracheal bifurcation suggested the possibility of pulmonary aspiration.
Imaging analysis
Activity seen in the region of the tracheal bifurcation, bronchi, or lungs in either hemithorax was indicative of aspiration (6). The entire 60-minute dynamic images were divided into 6 periods, 10 minutes/period. The time of the onset of abnormal activity in the bronchi, which was evidence of aspiration in each patient, was recorded and assigned to the corresponding period. The ratio of positive salivagrams every 10 minutes was recorded and compared.
Results
A total of 2,095 pediatric patients underwent the salivagram in our hospital. Based on the salivagram images, 398 patients (19.0%) had a positive result. All patients were suspicious of pulmonary aspiration, clinical symptoms including recurrent cough, chronic and recurrent pneumonia, or failure to thrive.
Among all 398 patients with evidence of aspiration, tracheobronchial tree activity could be seen in the first 10 minutes of the dynamic imaging in 184 of them (46.2%, 184/398) (Figure 1). The onset of the bronchial activity was seen between 10 and 20 minutes in 177 patients (44.5%, 177/398) (Figure 1). A total of 35 patients (8.8%, 35/398) had the onset of abnormal tracheobronchial tree activity in the 3rd period between 20 and 30 minutes (Figure 2). During the 4th period between 30 and 40 minutes, the onset of the aspiration occurred in only 2 patients (0.5%, 2/398) (Figures 3,4). All patients had the onset of aspiration in the first 40 minutes of the dynamic imaging.
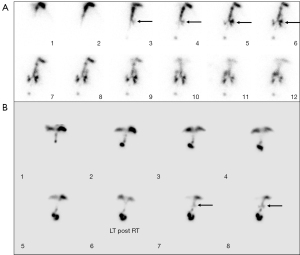



Discussion
In 1989, Heyman and Respondek first reported the application of a radionuclide salivagram for the diagnosis of pulmonary aspiration using 99mTc sulfur colloid droplets with a dose of 11.1 MBq and volume <0.1 mL on the tongue (5,8). The salivagram relies on the patient’s natural swallowing physiology by putting a small amount of radiopharmaceutical in the mouth and following the transit of the radioactivity to demonstrate whether the tracer is aspirated into the bronchi (9). Unlike other diagnostic methods for pulmonary aspiration, a salivagram does not involve a nonphysiologic bolus application of contrast agent or an IV injection of a radiotracer and is suitable for the pediatric patient (5). Previous studies reported that the salivagram had a higher detection rate for suspicion of aspiration, with 22.2% (6), 24.5% (10), 44.4% (8), and 56% (11). The difference in detection rate may be related to the underlying disease of the included patients. In these studies, some authors performed the salivagram for 60 minutes (6,11,12), some for 50 minutes (8), and some for 30 minutes (10). The original protocol for a salivagram required dynamic imaging for 60 minutes. The Society of Nuclear Medicine and Molecular Imaging guidelines recommend 30 to 60 minutes of dynamic imaging, and some institutions therefore only acquire 30-minute images. However, there is insufficient data to show whether shortened imaging times are adequate to detect aspiration. The present study demonstrated that 99.5% (396/398) of the salivagrams were positive in the first 30 minutes of the dynamic imaging, including 46.2% (184/398) in the first 10 minutes, 44.5% (177/398) in the second 10 minutes, and 8.8% (35/398) in the third 10 minutes. In the fourth 10 minutes, only 0.5% (2/398) of the salivagram showed positive results. No positive was detected in the fifth and sixth ten minutes, implying that the last 20 minutes of the original salivagram protocol were ineffective. In other words, the duration of the salivagram may be decreased to 40 or 30 minutes.
Aspiration is an important factor affecting the morbidity and mortality of respiratory diseases in pediatric patients (13). There are two types of pulmonary aspiration: anterograde and retrograde (14). Antegrade aspiration originates from the oropharynx, which usually contains a large amount of pathogenic bacteria oropharyngeal secretion. Aspiration of these oropharyngeal secretions can result in aspiration pneumonia (15). A radionuclide salivagram is an imaging method used to detect oropharyngeal secretion and saliva aspiration and to evaluate the antegrade route of aspiration. During the scan, the radionuclide follows the path of saliva, showing information about the aspiration of oral secretions, which is not related to the ingestion of solids or liquids. Retrograde aspiration is most commonly caused by gastric contents and occurs frequently in patients with severe gastroesophageal reflux (16). Aspiration of gastric contents into the respiratory airway can cause chemical damage (17). Gastroesophageal reflux scintigraphy is a radionuclide scan used to detect esophageal reflux and refluxate aspiration, mainly focusing on the evaluation of the retrograde route of pulmonary aspiration (18). The imaging tracer is fixed with food and ingested into the stomach during gastroesophageal reflux scintigraphy. During the gastroesophageal reflux scan, the radiotracer needs to travel retrograde from the stomach, which makes the distance to the lungs much longer. Therefore, we can speculate that gastroesophageal reflux scintigraphy needs more time compared with a salivagram. In our study, about 99.5% (396/398) of the salivagrams showed a positive result in the first 30 minutes of the dynamic imaging. In most studies, the duration of the gastroesophageal reflux scan was 60 minutes.
The goal of nuclear medicine scans is diagnostic accuracy in a reasonable amount of time. Various methods can be tried to obtain patient cooperation and immobilization. Holding the child in place may be sufficient for the youngest children, from neonates to 2 years old. Other methods, such as sleep deprivation prior to the scan and feeding the pediatric patient a bottle on the examination table, may be equally effective for most patients (19). Sandbags and entertainment such as video, music, and reading stories may also be effective for patients older than 4 or 5 years old. Frequently, if a child is afraid, it is unlikely that she or he will be immobilized unless her or his fears are relieved. Allowing parents to accompany or stay in the imaging room may help calm the patient’s fears. Allowing the patient to have a favorite toy is useful as well. Despite these efforts, some patients aged 1 to 5 years, as well as those with mental retardation or developmental delay, require sedation for most nuclear medicine scans. In this condition, the duration of the scan is very important. Most scans can be completed successfully if the salivagram is completed within 30 minutes. With the scan time increasing, the occurrence of mobilization would increase, which might influence the diagnostic accuracy (20). Moreover, pulmonary aspiration is a common problem in patients with brain injury (8). Causes of pulmonary aspiration include aspiration due to swallowing dysfunction and salivary aspiration (21). Risk factors for salivary aspiration include swallowing incoordination and absent laryngeal sensation (8). If these patients with brain injuries perform the salivagram with high quality, sedation may be required. However, several issues are identified. A variable success rate and a significant complication rate, including death, have been reported during the sedation. Moreover, the American Academy of Pediatrics’ guidelines recommend a systematic plan for the use of sedative medicine, including informed consent, pre-sedation health evaluation, and monitoring by a cardiopulmonary resuscitation-trained healthcare practitioner. Considering these factors, a short acquisition period is very important, not only for the patient but also for the physicians.
Protective reflexes to prevent aspiration of upper airway contents and an intact mucociliary clearance mechanism that allows rapid clearance of any foreign material entering the tracheobronchial tree play a key role in the prevention of lower respiratory tract infections (13). It is due to this normal airway defense mechanism that complications often do not occur. However, airway clearance mechanisms take time to operate (14). When pulmonary aspiration occurs, clearance may be accelerated due to the cough reflex. Wu et al. demonstrated that the likelihood of false-negative results increases with increasing time, and that selecting images at the 15-minute timepoint allows for diagnosis in 80% of cases with clearance of pulmonary aspiration and the lowest rate of missed diagnoses (22). In our study, when the time was shortened to 30 minutes, 99.5% of salivagrams were positive. Although only a very small percentage of cases were negative, this timepoint may have included some of the false-negative patients with clearance of pulmonary aspiration, which is a significant limitation of our study, and in the future we can further investigate the value of salivagrams in detecting pulmonary aspiration in a shorter time frame.
Conclusions
The originally described 60-minute dynamic imaging protocol of a salivagram can be safely shortened to 40 or even 30 minutes without a significantly decreased chance of detecting aspiration. Prolonged imaging is unnecessary.
Acknowledgments
The authors thank all the study investigators, clinicians, nurses, and technicians for dedicating their time and skills to the completion of this study.
Funding: This research was funded by the National Natural Science Foundation of China (Nos. 81971642, 82001861, 82102088, and 82272034), Capital’s Funds for Health Improvement and Research (No. 2020-2-2025), National Key Research and Development Plan (No. 2020YFC0122000).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-934/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work and for ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Institutional Review Board of Beijing Friendship Hospital, Capital Medical University approved the study, and the requirement for written informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Razia D, Mittal SK, Bansal S, Ravichandran R, Smith MA, Walia R, Bremner RM, Mohanakumar T, Tokman S. Lung Transplant Candidates With Pretransplant Gastroesophageal Reflux and Antibodies to Lung Self-antigens Have Shorter CLAD-free Survival After Transplant. Transplant Direct 2022;8:e1294. [Crossref] [PubMed]
- Tutor JD, Gosa MM. Dysphagia and aspiration in children. Pediatr Pulmonol 2012;47:321-37. [Crossref] [PubMed]
- Wu H, Zhao X. Pulmonary aspiration only increases the risk of right-sided pneumonia in children: comparison of salivagrams and chest radiographs. Nucl Med Commun 2018;39:505-10. [Crossref] [PubMed]
- Proesmans M. Respiratory illness in children with disability: a serious problem? Breathe (Sheff) 2016;12:e97-e103. [Crossref] [PubMed]
- Heyman S, Respondek M. Detection of pulmonary aspiration in children by radionuclide "salivagram". J Nucl Med 1989;30:697-9. [PubMed]
- Yang J, Codreanu I, Servaes S, Zhuang H. Radionuclide Salivagram and Gastroesophageal Reflux Scintigraphy in Pediatric Patients: Targeting Different Types of Pulmonary Aspiration. Clin Nucl Med 2015;40:559-63. [Crossref] [PubMed]
- Shao F, Zhao X, Toyama H, Ichihara T, Zhuang H, Zhao R, Kung BT, Ng KS, Zhang Z, Wu H. Semi-quantitative assessment optimized the grading of pulmonary aspiration on salivagram in children. Ann Nucl Med 2021;35:321-7. [Crossref] [PubMed]
- Kang Y, Chun MH, Lee SJ. Evaluation of salivary aspiration in brain-injured patients with tracheostomy. Ann Rehabil Med 2013;37:96-102. [Crossref] [PubMed]
- Lee DH, Kim JM, Lee Z, Park D. The effect of radionuclide solution volume on the detection rate of salivary aspiration in the radionuclide salivagram: A STROBE-compliant retrospective study. Medicine (Baltimore) 2018;97:e11729. [Crossref] [PubMed]
- Wu H, Zhao R. Image characteristics and classification of salivagram in the diagnosis of pulmonary aspiration in children. Nucl Med Commun 2017;38:617-22. [Crossref] [PubMed]
- Baikie G, South MJ, Reddihough DS, Cook DJ, Cameron DJ, Olinsky A, Ferguson E. Agreement of aspiration tests using barium videofluoroscopy, salivagram, and milk scan in children with cerebral palsy. Dev Med Child Neurol 2005;47:86-93. [Crossref] [PubMed]
- Drubach LA, Zurakowski D, Palmer EL 3rd, Tracy DA, Lee EY. Utility of salivagram in pulmonary aspiration in pediatric patients: comparison of salivagram and chest radiography. AJR Am J Roentgenol 2013;200:437-41. [Crossref] [PubMed]
- Adil E, Al Shemari H, Kacprowicz A, Perez J, Larson K, Hernandez K, Kawai K, Cowenhoven J, Urion D, Rahbar R. Evaluation and Management of Chronic Aspiration in Children With Normal Upper Airway Anatomy. JAMA Otolaryngol Head Neck Surg 2015;141:1006-11. [Crossref] [PubMed]
- Jadcherla SR, Hogan WJ, Shaker R. Physiology and pathophysiology of glottic reflexes and pulmonary aspiration: from neonates to adults. Semin Respir Crit Care Med 2010;31:554-60. [Crossref] [PubMed]
- Kovesi T. Aspiration Risk and Respiratory Complications in Patients with Esophageal Atresia. Front Pediatr 2017;5:62. [Crossref] [PubMed]
- Akbunar AT, Kiristioglu I, Alper E, Demiray H. Diagnosis of orotracheal aspiration using radionuclide salivagram. Ann Nucl Med 2003;17:415-6. [Crossref] [PubMed]
- Knight PR, Rutter T, Tait AR, Coleman E, Johnson K. Pathogenesis of gastric particulate lung injury: a comparison and interaction with acidic pneumonitis. Anesth Analg 1993;77:754-60. [Crossref] [PubMed]
- Elbl B, Birkenfeld B, Walecka A, Szymanowicz J, Listewnik M, Gwardyś A, Urasiński T. Upper gastrointestinal tract scintigraphy and ultrasonography in diagnosis of gastroesophageal reflux in children. Pol J Radiol 2011;76:63-7. [PubMed]
- Cui Y, Guo L, Mu Q, Cheng Q, Kang L, He Y, Tang M, Wu Q. Sleep deprivation did not enhance the success rate of chloral hydrate sedation for non-invasive procedural sedation in pediatric patients. PLoS One 2021;16:e0245338. [Crossref] [PubMed]
- Weiss S. Sedation of pediatric patients for nuclear medicine procedures. Semin Nucl Med 1993;23:190-8. [Crossref] [PubMed]
- Park D, Woo SB, Lee DH, Yu KJ, Cho JY, Kim JM, Lee Z. The Correlation Between Clinical Characteristics and Radionuclide Salivagram Findings in Patients With Brain Lesions: A Preliminary Study. Ann Rehabil Med 2017;41:915-23. [Crossref] [PubMed]
- Wu H, Zhao R, Zhao X. Use of Static Imaging as a Substitute for Conventional Dynamic Imaging for Salivagrams in Children. Clin Nucl Med 2019;44:532-4. [Crossref] [PubMed]


