
The value of quantitative plaque analysis based on coronary computed tomography angiography in predicting the percutaneous coronary intervention outcome of chronic total occlusion lesions
Introduction
Coronary chronic total occlusion (CTO) is defined as an occlusion with the absence of antegrade flow through the lesion [thrombolysis in myocardial infarction (TIMI) grade 0 flow] and with a presumed or documented duration of ≥3 months (1). It is characterized by a heavy atherosclerotic plaque burden within the coronary artery, resulting in complete occlusion of the vessel. The incidence of CTO has been reported to be as high as 18% to 46% in patients who are suspected of coronary artery disease (CAD) for coronary angiography (2-5). The vast majority of patients with CTO have symptoms such as arrhythmias and stable or variable angina pectoris. Successful percutaneous coronary intervention (PCI) for revascularizing CTO can reduce symptoms, improve quality of life, recover left ventricular function, and increase long-term survival rates compared with failed procedures (6,7). Despite the therapeutic benefits, PCI for CTO lesions is difficult due to its operational complexity. It has a relatively low success rate of about 59–70% compared to the 96% success rate for non-CTO-PCI (4,8,9). The overall operative success rate of CTO-PCI still needs to be improved.
Previously, the Multicenter Chronic Total Occlusion Registry of Japan (J-CTO) and Prospective Global Registry for the Study of Chronic Total Occlusion Intervention (PROGRESS CTO) scoring systems based on the morphological and anatomical characteristics from invasive coronary angiography (ICA) have been proposed to assess the PCI success rate and long-term prognosis. These 2 scoring systems exhibited moderate predictive performance, with an area under the curve (AUC) of about 0.63 (10). Unlike ICA, coronary computed tomography angiography (CCTA) is a noninvasive modality for diagnosing CAD with high sensitivity and specificity (11). Currently, CCTA is increasingly used for the preoperative evaluation of CTO-PCI because it has a unique ability to visualize the patent and occlusive portions of the CTO lesions. Based on morphologic parameters, such as a blunt stump, occlusion length, tortuous course, lesion of calcification, and the incremental attenuation of the proximal segment of the CTO lesion, CCTA was shown to predict the CTO-PCI outcome (12,13). However, the predictive performance of the morphologic parameters based on CCTA is still moderate, with the AUC ranging from 0.67 to 0.72 (14).
Besides morphologic analysis, CCTA can also be used to quantify coronary plaque features. Recently, certain quantitative and qualitative plaque characteristics derived from CCTA were shown to be associated with acute coronary syndrome (ACS) and adverse future cardiovascular events (15-17). It has been demonstrated that the peak radiological density of CTO segments represented by plaque with a high Hounsfield unit (HU; the hardest segment) on CCTA might be predictive of the CTO-PCI outcome (18). Nonetheless, many other plaque characteristics of CTO could be quantitatively measured from CCTA, such as plaque volume, burden, composition, and the degree of stenosis using dedicated cardiovascular postprocessing software (19). The quantitative plaque feature of low-density noncalcified plaque (LDNCP) burden quantified by this software was found to be associated with the incidence of myocardial ischemia in patients with suspected CAD (19). However, the performance of these quantitative plaque characteristics derived from CCTA in predicting CTO-PCI outcomes remains to be explored. Moreover, whether these quantitative plaque characteristics can outperform the conventional morphologic parameters remains unclear.
We hypothesized that using the quantitative plaque parameters obtained from preoperative CCTA could improve the success rate of CTO-PCI. In this study, the CCTA of patients with CTO confirmed by ICA was retrospectively analyzed. Morphologic and quantitative plaque parameters of CTO lesions were assessed, and their predictive performances were compared. The purpose of this study was to determine the value of plaque characteristics quantified from CCTA in predicting the CTO-PCI outcome. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-428/rc).
Methods
Patients
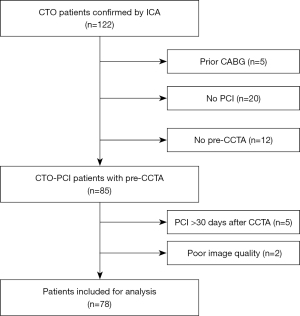
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Shantou Central Hospital, and individual consent for this retrospective analysis was waived. A total of 122 consecutive patients with CTO confirmed by ICA from Shantou Central Hospital between July 2016 and December 2018 were enrolled in this retrospective, case–control study. CTO diagnosis was defined as previously described (1). Patients were included if (I) they had CTO confirmed by ICA, and (II) this was their first attempt to recanalize the CTO lesions. Patients were excluded if (I) they had a prior history of coronary artery bypass graft surgery (CABG), (II) they had not received intervention revascularization of CTO, (III) they had not undergone CCTA before PCI, (IV) the interval between CCTA and PCI was more than 30 days, or (V) the image quality of the CCTA was poor. Finally, a total of 78 patients with 80 CTO lesions were included. Depending on the PCI outcome, the CTO lesions were grouped into PCI success and failure groups. A flowchart of the patient enrollment is shown in Figure 1. The demographic, clinical, and laboratory information were recorded from the electronic hospital database.
CCTA
All patients underwent CCTA on a dual-source CT (DSCT) scanner (Somatom Definition Flash, Siemens Healthineers, Erlangen, Germany). The scanning protocol included a noncontrast cardiac CT scanning and subsequent CCTA. The acquisition parameters were as previously described (20). The raw data were reconstructed into thin-slice images at the optimal cardiac diastolic and systolic phase with a section thickness of 0.75 mm, a reconstruction increment of 0.5 mm, and a smooth convolution kernel.
Image analysis
All thin-slice images were transferred to an image processing workstation (Syngo Via 40B; Siemens Healthineers). The CTO lesion was identified according to the complete contrast filling defect confirmed on axial and orthogonal cross-sectional images. Morphologic parameters of CTO were independently assessed by 2 experienced observers (G.D. and Z.C.) with 10 years of experience in cardiac diagnostic imaging who were blinded to the CTO-PCI outcomes. The morphologic parameters were stump morphology (sharp or blunt), occlusion length, tortuous course (angle >45°), CTO lesion calcium (present or absent), bridging collateral vessel (present or absent), retrograde collateral vessel (present or absent), and the appearance of the occluded distal segment (good: vessels well filled with contrast medium and without significant stenosis >50%; bad: poor visualization or stenosis >50%) and were assessed in a manner described previously (13,21). The CTO length was measured on CCTA from the proximal margin to the distal margin of the entire occluded segment.
Quantitative plaque analysis
All thin-slice images were anonymized and quantitatively analyzed using commercially available cardiovascular postprocessing software (Release 5.6.5; Circle Cardiovascular Imaging, Calgary, AB, Canada). After all thin-slice images were anonymized, image quality was first evaluated by 2 experienced radiologists (C.M and Y.T.) according to the guidelines of the Society of Cardiovascular Computed Tomography (22). The images were then quantitatively analyzed using the postprocessing software. The coronary artery tree was automatically extracted, and the centerlines were adjusted manually, if necessary. Two experienced radiologists (G.D. and Z.C.) who were blinded to the CTO-PCI outcomes manually identified the proximal and distal cross-sections of the CTO lesions as the starting and ending positions of plaque and arrived at a consensus. Afterward, the plaque volume, burden, distribution, plaque composition, and vascular stenosis degree were automatically analyzed to obtain the quantitative plaque parameters. These included the volume of total plaque, calcified plaque (CP), noncalcified plaque (NCP), and LDNCP; the burden of total plaque, CP, NCP, and LDNCP; diameter stenosis; remodeling index; plaque length; and contrast density drop.
The ICA and PCI procedure
The ICA was performed with the digital subtraction angiography (DSA) system (AlluraXper FD20, Philips, Best, the Netherlands). All CTO-PCI procedures were performed by 2 trained interventional cardiologists with more than 10 years of experience in CTO-PCI using the antegrade or retrograde approach according to standard practice. The antegrade approach is a wire-based or dissection technique that aims to pass through the CTO lesions from the proximal luminal segment to cross the distal CTO segment into the distal true lumen. The retrograde approach is a wire-based or dissection technique that aims to pass through the CTO lesions from the distal luminal segment to access the proximal CTO segment into the true lumen (1). The retrograde approach was attempted if the anterograde approach failed for more than 1 hour and if the approach through the collaterals was feasible. The outcome of PCI was defined as a success if the successful opening of total occlusion and restoring flow was achieved (<30% residual stenosis and TIMI ≥2 flow) (1,23). If the guidewire was consistently unable to pass through the occluded segment, or if severe dissection, perforation, and other complications of coronary artery occurred, the operation was terminated and defined as PCI failure (24).
Statistical analysis
All data, including CTA data and quantitative plaque characteristics, were collected between January 2019 and January 2021. Continuous variables are expressed as the mean ± standard deviation (SD) or median [interquartile range (IQR)]. Categorical variables are expressed as frequencies or percentages. Interobserver agreements of quantitative and categorical data were evaluated using the intraclass correlation coefficient (ICC) and Cohen’s kappa (κ) statistics, respectively. ICCs and κ values were interpreted as follows: 0.81–1.00, excellent; 0.61–0.80, good; 0.41–0.60, moderate; 0.20–0.40, fair; and <0.20, poor. The Student’s t test was used for normally distributed data. The Mann-Whitney test was used for nonnormally distributed data. The chi-squared test or Fisher exact test was used for categorical variables as appropriate. The statistical power was 0.986 based on the current sample and effect size, as calculated with PASS version 15 (NCSS LLC, Kaysville, UT, USA), indicating that the sample size provides sufficient power for the analysis. Univariate and multivariate logistic regression analyses were performed with the backward likelihood ratio method to identify predictors of CTO-PCI outcome, which were selected to construct the morphology-based model (morphologic analysis of CTO) and plaque-based model (quantitative analysis of the plaque). The predictive performance of individual models was compared using receiver operating characteristic (ROC) curve analysis with DeLong test. Statistical analysis was performed with SPSS 26.0 (IBM Corporation, Armonk, NY, USA) and MedCalc version 19.3.1. (MedCalc Software, Ostend, Belgium). A P value less than 0.05 was considered statistically significant.
Results
Clinical characteristics
A total of 78 patients with CTO were included in this study after 42 patients were excluded mainly due to a prior history of CABG (n=5), a lack of PCI for CTO (n=20), a lack of CCTA before PCI (n=12), or an interval between CCTA and PCI >30 days (n=5; Figure 1). The mean interval between CCTA and PCI was 5 days (range, 0–29 days), and the interval was within 1 week in 52 cases (66.67%). A total of 78 patients were divided into a PCI-success and PCI-failure group according to their PCI outcome. There were 47 (60.26%) patients in the PCI-success group (39 males, 8 females) and 31 (39.74%) in the PCI-failure group (25 males, 6 females). Among 31 patients in the PCI-failure group, 2 patients each had 2 lesions, and the remaining patients had 1 lesion each. There were no differences in clinical characteristics, including demographics, left ventricular ejection fraction (LVEF), risk factors, or a family history of coronary heart disease between the 2 groups (P>0.05). Detailed clinical characteristics are shown in Table 1.
Table 1
| Patient (n) | Total 78 (80 CTOs) | PCI-success 47 (47 CTOs) | PCI-failure 31 (33 CTOs) | P value |
|---|---|---|---|---|
| Age, years | 55.71±13.07 | 56.21±12.65 | 54.94±13.85 | 0.676 |
| Gender | 0.793 | |||
| Male | 64 (82.05%) | 39 (82.98%) | 25 (80.65%) | |
| Female | 14 (17.95%) | 8 (17.02%) | 6 (19.35%) | |
| BMI, kg/m2 | 26.28±3.05 | 26.16±2.90 | 26.46±3.29 | 0.677 |
| LVEF, % | 60.00 (55.00–75.00) | 62.00 (55.00–65.00) | 60.00 (53.00–63.00) | 0.192 |
| Hypertension | 57 (73.08%) | 35 (74.47%) | 22 (70.97%) | 0.733 |
| Diabetes mellitus | 24 (30.77%) | 15 (31.91%) | 9 (29.03%) | 0.787 |
| Hyperlipemia | 66 (84.62%) | 43 (91.49%) | 23 (74.19%) | 0.080 |
| Smoking | 54 (69.23%) | 30 (63.83%) | 24 (77.42%) | 0.203 |
| Drink | 30 (38.46%) | 20 (42.55%) | 10 (32.26%) | 0.360 |
| Family history of CHD | 9 (11.54%) | 5 (10.64%) | 4 (12.90%) | 1.000 |
Values are the mean ± standard deviation, median (interquartile range), or n (%). Variables were compared using the Student’s t test, Mann-Whitney test, chi-squared test, or Fisher exact test. A P value less than 0.05 indicated statistical significance. BMI, body mass index; CHD, coronary heart disease; CTO, coronary chronic total occlusion; LEVF, left ventricular ejection fraction; PCI, percutaneous coronary intervention.
ICA and PCI outcomes
ICA detected 80 CTO lesions in 78 patients. The right coronary artery (RCA) was the most common location of CTO (44/80, 55.00%), followed by the left anterior descending artery (LAD; 30/80, 37.50%) and the left circumflex artery (LCX; 6/80, 7.50%). PCI was successfully achieved for 47 lesions of the 80 CTO lesions (47/80, 58.75%) and PCI failed in 33 lesions (33/80, 41.25%). The antegrade approach was applied to 72 lesions (72/80, 90.00%), and the retrograde approach was adopted in 8 lesions (8/80, 10.00%). Failure to pass through the guidewire was a predominant cause for PCI failure (31/33, 93.93%). PCI was terminated in 2 patients due to severe procedure-related coronary dissection. No other serious complications, such as wire perforation, cardiac tamponade, or acute myocardial infarction, were noted in any of the patients.
CCTA morphologic parameters
The morphologic parameters of CTO in the PCI-success group and the PCI-failure group are shown in Table 2. Univariate logistic regression analysis showed the blunt stump was the only morphologic parameter associated with PCI outcome [odds ratio (OR): 10.81; P<0.001]. Other morphologic parameters, including the lesion site, occlusion length, torturous course, CTO lesion calcium, bridging collateral vessels, retrograde collateral vessels, and good visualization of the occluded distal segment, were not significantly different between the 2 groups (P>0.05). Occlusion lengths categorized as either <20 mm or ≥20 mm according to the consensus of the Euro CTO Club (25) were also not significantly different between the 2 groups (P>0.05). The interobserver agreements on CCTA morphological parameters were excellent for all lesions (κ=0.86–0.90).
Table 2
| PCI-success (47 CTOs) | PCI-failure (33 CTOs) | OR (95% CI) | P value | |
|---|---|---|---|---|
| Lesion site | 0.529 | |||
| RCA | 24 (51.06%) | 20 (60.61%) | – | |
| LAD | 20 (42.55%) | 10 (30.30%) | 0.600 (0.229–1.573) | 0.299 |
| LCX | 3 (6.38%) | 3 (9.09%) | 1.200 (0.218–6.613) | 0.834 |
| Occlusion length (mm) | 24.00 (15.00–35.00) | 24.00 (18.00–34.50) | 0.999 (0.996–1.034) | 0.976 |
| Occlusion length ≥20 mm | 29 (61.70%) | 23 (69.70%) | 1.428 (0.554–3.681) | 0.461 |
| Blunt stump | 3 (6.38%) | 14 (42.42%) | 10.807 (2.779–42.025) | <0.001* |
| Torturous course | 4 (8.51%) | 4 (12.12%) | 1.483 (0.343–6.409) | 0.598 |
| CTO lesion calcium | 32 (68.09%) | 27 (81.82%) | 2.109 (0.719–6.189) | 0.174 |
| Bridging collateral vessel | 3 (6.38%) | 3 (9.09%) | 1.467 (0.277–7.762) | 0.652 |
| Retrograde collateral vessel | 46 (97.87%) | 30 (90.91%) | 0.217 (0.022–2.189) | 0.195 |
| Good visualization of the occluded distal segment | 25 (53.19%) | 17 (51.52%) | 0.935 (0.383–2.280) | 0.883 |
Values are the median (interquartile range) or n (%). Variables were compared using univariate logistic regression analysis. *, A P value less than 0.05 indicated statistical significance. CI, confidence interval; CTO, coronary chronic total occlusion; LAD, left anterior descending artery; LCX, left circumflex artery; OR, odds ratio; RCA, right coronary artery; PCI, percutaneous coronary intervention.
Quantitative plaque parameters
The quantitative characteristics of plaques in CTO lesions in the PCI-success group and the PCI-failure group are shown in Table 3. Total plaque volume, CP volume, NCP volume, LDNCP volume, and plaque length were significantly lower in the PCI-success group than in the PCI-failure group (P<0.001–0.02).
Table 3
| Plaque characteristics | PCI-success (47 CTOs) | PCI-failure (33 CTOs) | OR (95% CI) | P value |
|---|---|---|---|---|
| The volume of plaque (mm3) | ||||
| Total plaque volume | 230.51 (172.73–277.64) | 538.24 (396.89–690.15) | 1.024 (1.011–1.036) | <0.001* |
| CP volume | 2.51 (0–14.97) | 10.56 (1.40–36.40) | 1.015 (1.001–1.029) | 0.041* |
| NCP volume | 214.98 (153.76–267.37) | 484.46 (310.98–678.66) | 1.018 (1.008–1.027) | <0.001* |
| LDNCP volume | 42.71 (28.32–71.26) | 146.01 (78.73–242.26) | 1.023 (1.012–1.035) | <0.001* |
| The burden of plaque (%) | ||||
| Total plaque burden | 63.74 (48.97–74.21) | 63.78 (52.63–76.60) | 3.048 (0.261–35.605) | 0.374 |
| CP burden | 0.73 (0–3.72) | 1.12 (0.26–4.26) | 34.962 (0.026–4.733E4) | 0.334 |
| NCP burden | 61.49 (41.04–73.88) | 60.12 (41.77–74.60) | 1.729 (0.204–14.665) | 0.616 |
| LDNCP burden | 13.66 (6.91–19.51) | 17.28 (10.98–20.59) | 87.344 (0.409–1.865E4) | 0.102 |
| Plaque ratio (%) | ||||
| NCP | 98.84 (91.48–100.00) | 97.53 (94.02–99.37) | 0.382 (0.015–9.784) | 0.561 |
| LDNCP | 22.79 (14.92–29.03) | 25.62 (19.06–39.85) | 3.681 (0.344–39.419) | 0.281 |
| Remodeling index | 1.36 (1.18–1.53) | 1.31 (1.18–1.48) | 0.363 (0.063–2.094) | 0.257 |
| Plaque length (mm) | 27.62 (21.42–39.77) | 61.43 (43.40–79.34) | 1.099 (1.053–1.147) | <0.001* |
| Contrast density drop (%) | 100.00 (74.23–100.00) | 100.00 (90.33–100.00) | 1.210 (0.204–7.188) | 0.834 |
Values are the median (interquartile range). Variables were compared using univariate logistic regression analysis; *, A P value less than 0.05 indicated statistical significance. CI, confidence interval; CP, calcified plaque; CTO, coronary chronic total occlusion; LDNCP, low-density noncalcified plaque; NCP, noncalcified plaque; OR, odds ratio; PCI, percutaneous coronary intervention.
Predictive performances of morphologic parameters and quantitative characteristics of plaques
Among the 5 quantitative parameters of total plaque volume, CP volume, NCP volume, LDNCP volume, and plaque length, multivariate logistic regression analysis showed that NCP volume, CP volume, and plaque length were independent predictors of CTO-PCI outcome (Table 4). When the value of the NCP volume, CP volume, and plaque length was raised by 1 unit, the odds of PCI failure increased by 1.018, 1.026, and 1.058, respectively. The regression equation was as follows: logit(P) = 8.821 – 0.018 × (NCP volume) – 0.026 × (CP volume) – 0.056 × (plaque length), where logit(P) is the probability that the plaque-based model combining NCP volume with CP volume and plaque length can predict CTO-PCI outcome.
Table 4
| Characteristics | β | OR | 95% CI | P value |
|---|---|---|---|---|
| NCP volume | 0.018 | 1.018 | 1.006–1.030 | 0.003* |
| CP volume | 0.026 | 1.026 | 1.001–1.053 | 0.049* |
| Plaque length | 0.056 | 1.058 | 1.003–1.115 | 0.037* |
*, A P value less than 0.05 indicated statistical significance. CI, confidence interval; CP, calcified plaque; CTO, coronary chronic total occlusion; NCP, noncalcified plaque; OR, odds ratio; PCI, percutaneous coronary intervention.
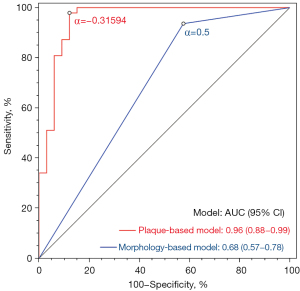
ROC analysis showed that the AUC of the morphology-based model (blunt stump) was 0.68 in predicting the PCI outcome (sensitivity, 93.62%; specificity, 42.42%; accuracy, 72.50%; Table 5). At the optimal predictive probability threshold of –0.31594, the AUC of the plaque-based model was 0.96, with a sensitivity of 97.87%, a specificity of 87.88%, and an accuracy of 93.75% (Table 5). The AUC of the plaque-based model was higher than that of the morphology-based model (0.96 vs. 0.68; P<0.001; Figure 2).
Table 5
| Model | Threshold | AUC | Sensitivity (%) | Specificity (%) | Accuracy (%) | P value |
|---|---|---|---|---|---|---|
| Morphology-based model | 0.5 | 0.68 (0.57–0.78) | 93.62 (82.46–98.66) | 42.42 (25.48–60.78) | 72.50 (61.38–81.90) | 0.006 |
| Plaque-based model | –0.31594 | 0.96 (0.88–0.99) | 97.87 (88.71–99.95) | 87.88 (71.80–96.60) | 93.75 (86.01–97.94) | <0.001 |
Data in parentheses are 95% CIs. The morphology-based model was composed of the blunt stump. The plaque-based model consisted of noncalcified plaque volume, calcified plaque volume, and plaque length. AUC, area under the curve; CI, confidence interval; CTO, coronary chronic total occlusion; ROC, receiver operating characteristic; PCI, percutaneous coronary intervention.
Discussion
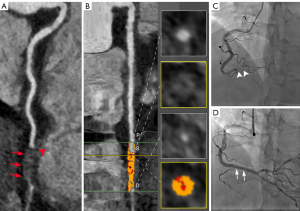
Our studies demonstrated that quantitative plaque parameters of the CTO lesions, including NCP volume, CP volume, and plaque length, were independent predictors of PCI outcome in patients with CTO. Moreover, the combined quantitative plaque parameters improved the predictive performance of the PCI outcome in patients with CTO compared to CCTA morphologic parameters (Figure 3).
CTO of the coronary artery remains a complex scenario for PCI. Despite remarkable technological advances and improvements in interventional operations, the success rate of PCI for the recanalization of CTO is considerably lower than that of nonoccluded vessels. In our study, the success rate of CTO-PCI was approximately 58.75% (47/80). Comparatively, the success rate of CTO-PCI in our study was slightly lower than that reported previously (4,8). The degree of calcification, negative remodeling, and the presence of necrotic core along the plaque are associated with the success rate of PCI for CTO (26). Thus, the morphologic features of CTO might have affected the success rate of PCI in the patients enrolled. In addition, the restricted availability of dedicated equipment and the limited case numbers from a single center may be the other reasons for our study’s relatively lower success rate of CTO-PCI.
The primary purpose of preoperative CCTA in CTO patients is to identify lesion features to more accurately predict PCI outcomes, thereby helping surgeons select appropriate candidates for PCI treatment. Preoperative assessment of morphologic characteristics of CTO is considered helpful in guiding coronary intervention (Figure 4) (12,13). In our study, a blunt stump was the only significant morphologic parameter associated with PCI failure. This finding is similar to the findings of previous studies (27). This result probably occurs because a blunt stump at the entry of the occlusion prevents the guidewire from entering the occluded segment, while a tapered stump is easier for the guidewire to pass through (Figure 4) (28). However, our study showed that the ability of the blunt stump to predict the PCI outcome was moderate (AUC 0.68). It is difficult to accurately predict the CTO-PCI outcome based on the appearance of the blunt stump alone.
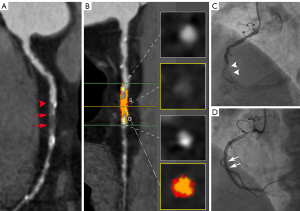
In addition, other morphologic features, such as occlusion length, tortuous course (angle >45°), CTO lesion calcification, bridging collateral vessel, retrograde collateral vessel, and the appearance of the occluded distal segment, had limited predictive values due to the similar prevalence of these signs in the PCI-success patients and PCI-failure patients. Calcification of more than 50% of the vessel’s cross-section, usually considered a predictor of PCI failure (29-31), was not included in this study. The reason for this exclusion was that calcification produces a blooming and beam-hardening artifact on CCTA images, which could cause enlargement of the appearance of calcification (32,33).
Other than morphological features, some quantitative plaque characteristics derived from CCTA are also considered to be associated with the outcome of PCI in CTO patients (18). Despite the association, manual quantitative analysis of CCTA data is time-intensive and subject to interobserver variability, and these parameters have yet to be systematically evaluated in clinical practice. Recent progress in CT image postprocessing software enables quantitative analysis of the coronary artery stenosis, degree, and plaque characteristics, which saves time and improves the accuracy and repeatability of the CTO diagnosis (19,34).
Existing studies have performed quantitative plaque measurement of the CTO lesion based on CT and have analyzed the relationship between the plaque characteristics and PCI outcome (18). The results showed high segmental radiologic density (>139 HU) to be an independent predictor of CTO-PCI failure (18). In our study, plaque characteristics were automatically analyzed in patients with CTO. Our results showed that total plaque volume, CP volume, NCP volume, LDNCP volume, and plaque length of CTO lesions differed between the PCI-success group and the PCI-failure group. Multivariate logistic analysis showed that NCP volume, CP volume, and plaque length were associated with the CTO-PCI outcomes, with an OR ranging from 1.018 to 1.058. These findings suggest that the increase of plaque volume and length and the hardening of plaque likely prevent the guidewire from passing through the CTO lesions which results in PCI failure. The guidewire’s successful passage through the coronary lesion is supposed to be the premise of successful coronary intervention (35,36). Histopathological studies have also suggested that hard or fibrocalcific CTO lesions are difficult for the guidewire to pass through or dilate, compared with predominantly soft or lipid-laden lesions (26). Plaque characteristics quantitatively measured from CCTA are likely to reveal the nature of the CTO lesions and be predictive of PCI outcome.
In our study, quantitative plaque characteristics combining NCP volume with CP volume and plaque length achieved good performance (AUC 0.96), one better than that of a morphology-based model using a blunt stump (AUC 0.68). This performance was also superior to those of previously reported morphological prediction tools, such as the multicenter RECHARGE (Registry of CrossBoss and Hybrid Procedures In France, the Netherlands, Belgium, and the United Kingdom) based on CCTA, the J-CTO based on CCTA, the CT-RECTOR (Computed Tomography Registry of Chronic Total Occlusion Revascularization) score, and the KCCT (Korean Multicenter Coronary chronic total occlusion CT Registry) score (AUC 0.718–0.882) (14,37-39). The good predictive performance of combining NCP volume with CP volume and plaque length can be attributed to their direct mirroring of the plaque component and plaque burden, which are essential pathological factors related to the feasibility of crossing the CTO lesion with a guidewire (40). Collectively, the quantitative characteristics of the plaque in CTO lesions are better than are morphologic parameters in predicting the PCI outcome.
In recent years, radiomics and artificial intelligence (AI) have been used to detect CAD (41). Radiomics allows accurate quantitative analysis of plaque morphology and composition to be performed using CCTA. Studies have demonstrated that the radiomics features derived from CCTA can help identify vulnerable plaques and discriminate between napkin-ring sign and non–napkin-ring sign plaques (42,43). AI has been shown to have outstanding performance in certain cardiovascular image analysis tasks, such as automatic detection and classification of coronary plaques (44). The use of AI has several advantages, such as the reduction in image analysis time and the enhancement of tasks involved in precision medicine (44). However, quantitative analysis of plaque characteristics of CTO based on radiomics or AI approaches remains inadequate (45). More efforts are needed to determine the role of radiomics and AI in the reliable quantification of atherosclerotic plaques.
Study limitations
Our study has several limitations. First, this was a single-center and retrospective study that might have had patient selection biases. Further studies with larger sample sizes are warranted. Second, the quantitative plaque characteristics provided by the plaque analysis software were not validated with other imaging modalities, such as intravascular ultrasound (IVUS). However, a previous study has shown good to excellent agreement between CCTA plaque quantitative analysis and IVUS (r=0.56–0.94) (34). Third, we used dedicated software to quantitatively assess the plaque characteristics based on the CCTA. This software is commercially available and has been widely used in cardiovascular imaging. The measurement of quantitative plaque characteristics was semiautomatic, and only minimal manual intervention was introduced in our study. Thus, we believe that the results of our study have good extrapolation potential. However, the quantitative analysis also has some shortcomings, such as requirements for additional technical work, specific postprocessing software, and the greater time needed compared to conventional evaluation, which might restrict its availability in clinical practice.
Conclusions
Our study showed that the combination of NCP volume, CP volume, and plaque length had a superior performance to that of the CCTA morphologic parameter in predicting PCI outcomes for patients with CTO. Quantitative plaque analysis based on CCTA outperformed the morphologic analysis in predicting the CTO-PCI outcome and can be used to predict the PCI outcome in patients with CTO lesions.
Acknowledgments
We thank all our colleagues from the cardiology and radiology departments of Shantou Central Hospital and the patients for participating in the study. G. Du would like to thank his supervisor Prof. Youyou Yang for his constant encouragement and guidance.
Funding: This work was supported by the Shantou Medical Science and Technology Planning Project ([(2015)123]; No. 20150406).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-428/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-428/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ybarra LF, Rinfret S, Brilakis ES, Karmpaliotis D, Azzalini L, Grantham JA, et al. Definitions and Clinical Trial Design Principles for Coronary Artery Chronic Total Occlusion Therapies: CTO-ARC Consensus Recommendations. Circulation 2021;143:479-500. [Crossref] [PubMed]
- Jeroudi OM, Alomar ME, Michael TT, El Sabbagh A, Patel VG, Mogabgab O, Fuh E, Sherbet D, Lo N, Roesle M, Rangan BV, Abdullah SM, Hastings JL, Grodin J, Banerjee S, Brilakis ES. Prevalence and management of coronary chronic total occlusions in a tertiary Veterans Affairs hospital. Catheter Cardiovasc Interv 2014;84:637-43. [Crossref] [PubMed]
- Tsai TT, Stanislawski MA, Shunk KA, Armstrong EJ, Grunwald GK, Schob AH, Valle JA, Alfonso CE, Nallamothu BK, Ho PM, Rumsfeld JS, Brilakis ES. Contemporary Incidence, Management, and Long-Term Outcomes of Percutaneous Coronary Interventions for Chronic Coronary Artery Total Occlusions: Insights From the VA CART Program. JACC Cardiovasc Interv 2017;10:866-75. [Crossref] [PubMed]
- Fefer P, Knudtson ML, Cheema AN, Galbraith PD, Osherov AB, Yalonetsky S, Gannot S, Samuel M, Weisbrod M, Bierstone D, Sparkes JD, Wright GA, Strauss BH. Current perspectives on coronary chronic total occlusions: the Canadian Multicenter Chronic Total Occlusions Registry. J Am Coll Cardiol 2012;59:991-7. [Crossref] [PubMed]
- Råmunddal T, Hoebers LP, Henriques JP, Dworeck C, Angerås O, Odenstedt J, Ioanes D, Olivecrona G, Harnek J, Jensen U, Aasa M, Jussila R, James S, Lagerqvist B, Matejka G, Albertsson P, Omerovic E. Chronic total occlusions in Sweden--a report from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR). PLoS One 2014;9:e103850. [Crossref] [PubMed]
- Christakopoulos GE, Christopoulos G, Carlino M, Jeroudi OM, Roesle M, Rangan BV, Abdullah S, Grodin J, Kumbhani DJ, Vo M, Luna M, Alaswad K, Karmpaliotis D, Rinfret S, Garcia S, Banerjee S, Brilakis ES. Meta-analysis of clinical outcomes of patients who underwent percutaneous coronary interventions for chronic total occlusions. Am J Cardiol 2015;115:1367-75. [Crossref] [PubMed]
- Sapontis J, Salisbury AC, Yeh RW, Cohen DJ, Hirai T, Lombardi W, et al. Early Procedural and Health Status Outcomes After Chronic Total Occlusion Angioplasty: A Report From the OPEN-CTO Registry (Outcomes, Patient Health Status, and Efficiency in Chronic Total Occlusion Hybrid Procedures). JACC Cardiovasc Interv 2017;10:1523-34. [Crossref] [PubMed]
- Brilakis ES, Banerjee S, Karmpaliotis D, Lombardi WL, Tsai TT, Shunk KA, Kennedy KF, Spertus JA, Holmes DR Jr, Grantham JA. Procedural outcomes of chronic total occlusion percutaneous coronary intervention: a report from the NCDR (National Cardiovascular Data Registry). JACC Cardiovasc Interv 2015;8:245-53. [Crossref] [PubMed]
- Hannan EL, Zhong Y, Jacobs AK, Stamato NJ, Berger PB, Walford G, Sharma S, Venditti FJ, King SB 3rd. Patients With Chronic Total Occlusions Undergoing Percutaneous Coronary Interventions: Characteristics, Success, and Outcomes. Circ Cardiovasc Interv 2016;9:e003586. [Crossref] [PubMed]
- Forouzandeh F, Suh J, Stahl E, Ko YA, Lee S, Joshi U, Sabharwal N, Almuwaqqat Z, Gandhi R, Lee HS, Ahn SG, Gogas BD, Douglas JS, Robertson G, Jaber W, Karmpaliotis D, Brilakis ES, Nicholson WJ, King SB 3rd, Samady H. Performance of J-CTO and PROGRESS CTO Scores in Predicting Angiographic Success and Long-term Outcomes of Percutaneous Coronary Interventions for Chronic Total Occlusions. Am J Cardiol 2018;121:14-20. [Crossref] [PubMed]
- Hamon M, Biondi-Zoccai GG, Malagutti P, Agostoni P, Morello R, Valgimigli M, Hamon M. Diagnostic performance of multislice spiral computed tomography of coronary arteries as compared with conventional invasive coronary angiography: a meta-analysis. J Am Coll Cardiol 2006;48:1896-910. [Crossref] [PubMed]
- Wang N, Fulcher J, Abeysuriya N, Adams M, Lal S. Predictors of successful chronic total occlusion percutaneous coronary interventions: a systematic review and meta-analysis. Heart 2018;104:517-24. [Crossref] [PubMed]
- Brilakis ES, Mashayekhi K, Tsuchikane E, Abi Rafeh N, Alaswad K, Araya M, et al. Guiding Principles for Chronic Total Occlusion Percutaneous Coronary Intervention. Circulation 2019;140:420-33. [Crossref] [PubMed]
- Li J, Wang R, Tesche C, Schoepf UJ, Pannell JT, He Y, Huang R, Chen Y, Li J, Song X CT. Angiography-Derived RECHARGE Score Predicts Successful Percutaneous Coronary Intervention in Patients with Chronic Total Occlusion. Korean J Radiol 2021;22:697-705. [Crossref] [PubMed]
- Ferencik M, Liu T, Mayrhofer T, Puchner SB, Lu MT, Maurovich-Horvat P, Pope JH, Truong QA, Udelson JE, Peacock WF, White CS, Woodard PK, Fleg JL, Nagurney JT, Januzzi JL, Hoffmann U. hs-Troponin I Followed by CT Angiography Improves Acute Coronary Syndrome Risk Stratification Accuracy and Work-Up in Acute Chest Pain Patients: Results From ROMICAT II Trial. JACC Cardiovasc Imaging 2015;8:1272-81. [Crossref] [PubMed]
- Liu T, Maurovich-Horvat P, Mayrhofer T, Puchner SB, Lu MT, Ghemigian K, Kitslaar PH, Broersen A, Pursnani A, Hoffmann U, Ferencik M. Quantitative coronary plaque analysis predicts high-risk plaque morphology on coronary computed tomography angiography: results from the ROMICAT II trial. Int J Cardiovasc Imaging 2018;34:311-9. [Crossref] [PubMed]
- Puchner SB, Liu T, Mayrhofer T, Truong QA, Lee H, Fleg JL, Nagurney JT, Udelson JE, Hoffmann U, Ferencik M. High-risk plaque detected on coronary CT angiography predicts acute coronary syndromes independent of significant stenosis in acute chest pain: results from the ROMICAT-II trial. J Am Coll Cardiol 2014;64:684-92. [Crossref] [PubMed]
- Choi JH, Song YB, Hahn JY, Choi SH, Gwon HC, Cho JR, Jang Y, Choe Y. Three-dimensional quantitative volumetry of chronic total occlusion plaque using coronary multidetector computed tomography. Circ J 2011;75:366-75. [Crossref] [PubMed]
- Liu T, Yuan X, Wang C, Sun M, Jin S, Dai X. Quantification of plaque characteristics detected by dual source computed tomography angiography to predict myocardial ischemia as assessed by single photon emission computed tomography myocardial perfusion imaging. Quant Imaging Med Surg 2019;9:711-21. [Crossref] [PubMed]
- Yuan M, Wu H, Li R, Yu M, Dai X, Zhang J. The value of quantified plaque analysis by dual-source coronary CT angiography to detect vulnerable plaques: a comparison study with intravascular ultrasound. Quant Imaging Med Surg 2020;10:668-77. [Crossref] [PubMed]
- Opolski MP. Cardiac Computed Tomography for Planning Revascularization Procedures. J Thorac Imaging 2018;33:35-54. [Crossref] [PubMed]
- Leipsic J, Abbara S, Achenbach S, Cury R, Earls JP, Mancini GJ, Nieman K, Pontone G, Raff GL. SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr 2014;8:342-58. [Crossref] [PubMed]
- Gutiérrez-Barrios A, Gheorghe L, Camacho-Freire S, Valencia-Serrano F, Cañadas-Pruaño D, Calle-Pérez G, Alarcón de la Lastra I, Silva E, García-Molinero D, Agarrado-Luna A, Zayas-Ruedas R, Vázquez-García R, Serra A. Primary Angioplasty in a Catastrophic Presentation: Acute Left Main Coronary Total Occlusion-The ATOLMA Registry. J Interv Cardiol 2020;2020:5246504. [Crossref] [PubMed]
- Mattichak SJ, Dixon SR, Shannon F, Boura JA, Safian RD. Failed percutaneous coronary intervention: a decade of experience in 21,000 patients. Catheter Cardiovasc Interv 2008;71:131-7. [Crossref] [PubMed]
- Di Mario C, Werner GS, Sianos G, Galassi AR, Büttner J, Dudek D, Chevalier B, Lefevre T, Schofer J, Koolen J, Sievert H, Reimers B, Fajadet J, Colombo A, Gershlick A, Serruys PW, Reifart N. European perspective in the recanalisation of Chronic Total Occlusions (CTO): consensus document from the EuroCTO Club. EuroIntervention 2007;3:30-43. [PubMed]
- Srivatsa SS, Edwards WD, Boos CM, Grill DE, Sangiorgi GM, Garratt KN, Schwartz RS, Holmes DR Jr. Histologic correlates of angiographic chronic total coronary artery occlusions: influence of occlusion duration on neovascular channel patterns and intimal plaque composition. J Am Coll Cardiol 1997;29:955-63. [Crossref] [PubMed]
- Opolski MP, Achenbach S, Schuhbäck A, Rolf A, Möllmann H, Nef H, Rixe J, Renker M, Witkowski A, Kepka C, Walther C, Schlundt C, Debski A, Jakubczyk M, Hamm CW. Coronary computed tomographic prediction rule for time-efficient guidewire crossing through chronic total occlusion: insights from the CT-RECTOR multicenter registry (Computed Tomography Registry of Chronic Total Occlusion Revascularization). JACC Cardiovasc Interv 2015;8:257-67. [Crossref] [PubMed]
- Chen YH, Leong WS, Lin MS, Huang CC, Hung CS, Li HY, Chan KK, Yeh CF, Chiu MJ, Kao HL. Predictors for Successful Endovascular Intervention in Chronic Carotid Artery Total Occlusion. JACC Cardiovasc Interv 2016;9:1825-32. [Crossref] [PubMed]
- Soon KH, Cox N, Wong A, Chaitowitz I, Macgregor L, Santos PT, Selvanayagam JB, Farouque HM, Rametta S, Bell KW, Lim YL. CT coronary angiography predicts the outcome of percutaneous coronary intervention of chronic total occlusion. J Interv Cardiol 2007;20:359-66. [Crossref] [PubMed]
- García-García HM, van Mieghem CA, Gonzalo N, Meijboom WB, Weustink AC, Onuma Y, Mollet NR, Schultz CJ, Meliga E, van der Ent M, Sianos G, Goedhart D, den Boer A, de Feyter P, Serruys PW. Computed tomography in total coronary occlusions (CTTO registry): radiation exposure and predictors of successful percutaneous intervention. EuroIntervention 2009;4:607-16. [Crossref] [PubMed]
- Cho JR, Kim YJ, Ahn CM, Moon JY, Kim JS, Kim HS, Kim MK, Ko YG, Choi D, Chung N, Choe KO, Shim WH, Cho SY, Jang Y. Quantification of regional calcium burden in chronic total occlusion by 64-slice multi-detector computed tomography and procedural outcomes of percutaneous coronary intervention. Int J Cardiol 2010;145:9-14. [Crossref] [PubMed]
- Sarwar A, Rieber J, Mooyaart EA, Seneviratne SK, Houser SL, Bamberg F, Raffel OC, Gupta R, Kalra MK, Pien H, Lee H, Brady TJ, Hoffmann U. Calcified plaque: measurement of area at thin-section flat-panel CT and 64-section multidetector CT and comparison with histopathologic findings. Radiology 2008;249:301-6. [Crossref] [PubMed]
- Li P, Xu L, Yang L, Wang R, Hsieh J, Sun Z, Fan Z, Leipsic JA. Blooming Artifact Reduction in Coronary Artery Calcification by A New De-blooming Algorithm: Initial Study. Sci Rep 2018;8:6945. [Crossref] [PubMed]
- Nakanishi R, Motoyama S, Leipsic J, Budoff MJ. How accurate is atherosclerosis imaging by coronary computed tomography angiography? J Cardiovasc Comput Tomogr 2019;13:254-60. [Crossref] [PubMed]
- Morino Y, Abe M, Morimoto T, Kimura T, Hayashi Y, Muramatsu T, Ochiai M, Noguchi Y, Kato K, Shibata Y, Hiasa Y, Doi O, Yamashita T, Hinohara T, Tanaka H, Mitsudo K. J-CTO Registry Investigators. Predicting successful guidewire crossing through chronic total occlusion of native coronary lesions within 30 minutes: the J-CTO (Multicenter CTO Registry in Japan) score as a difficulty grading and time assessment tool. JACC Cardiovasc Interv 2011;4:213-21. [Crossref] [PubMed]
- Staruch AD, Opolski MP, Slomka PJ, Staruch M, Kepka C, Witkowski A, Kruk M, Dey D. Automated Quantitative Plaque Analysis for Discrimination of Coronary Chronic Total Occlusion and Subtotal Occlusion in Computed Tomography Angiography. J Thorac Imaging 2016;31:367-72. [Crossref] [PubMed]
- Tan Y, Zhou J, Zhang W, Zhou Y, Du L, Tian F, Guo J, Chen L, Cao F, Chen Y. Comparison of CT-RECTOR and J-CTO scores to predict chronic total occlusion difficulty for percutaneous coronary intervention. Int J Cardiol 2017;235:169-75. [Crossref] [PubMed]
- Li Y, Xu N, Zhang J, Li M, Lu Z, Wei M, Lu B, Zhang Y. Procedural success of CTO recanalization: Comparison of the J-CTO score determined by coronary CT angiography to invasive angiography. J Cardiovasc Comput Tomogr 2015;9:578-84. [Crossref] [PubMed]
- Yu CW, Lee HJ, Suh J, Lee NH, Park SM, Park TK, Yang JH, Song YB, Hahn JY, Choi SH, Gwon HC, Lee SH, Choe YH, Kim SM, Choi JH. Coronary Computed Tomography Angiography Predicts Guidewire Crossing and Success of Percutaneous Intervention for Chronic Total Occlusion: Korean Multicenter CTO CT Registry Score as a Tool for Assessing Difficulty in Chronic Total Occlusion Percutaneous Coronary Intervention. Circ Cardiovasc Imaging 2017;10:e005800. [Crossref] [PubMed]
- Irving J. CTO pathophysiology: how does this affect management? Curr Cardiol Rev 2014;10:99-107. [Crossref] [PubMed]
- Infante T, Cavaliere C, Punzo B, Grimaldi V, Salvatore M, Napoli C. Radiogenomics and Artificial Intelligence Approaches Applied to Cardiac Computed Tomography Angiography and Cardiac Magnetic Resonance for Precision Medicine in Coronary Heart Disease: A Systematic Review. Circ Cardiovasc Imaging 2021;14:1133-46. [Crossref] [PubMed]
- Kolossváry M, Karády J, Szilveszter B, Kitslaar P, Hoffmann U, Merkely B, Maurovich-Horvat P. Radiomic Features Are Superior to Conventional Quantitative Computed Tomographic Metrics to Identify Coronary Plaques With Napkin-Ring Sign. Circ Cardiovasc Imaging 2017;10:e006843. [Crossref] [PubMed]
- Kolossváry M, Park J, Bang JI, Zhang J, Lee JM, Paeng JC, Merkely B, Narula J, Kubo T, Akasaka T, Koo BK, Maurovich-Horvat P. Identification of invasive and radionuclide imaging markers of coronary plaque vulnerability using radiomic analysis of coronary computed tomography angiography. Eur Heart J Cardiovasc Imaging 2019;20:1250-8. [Crossref] [PubMed]
- Dey D, Slomka PJ, Leeson P, Comaniciu D, Shrestha S, Sengupta PP, Marwick TH. Artificial Intelligence in Cardiovascular Imaging: JACC State-of-the-Art Review. J Am Coll Cardiol 2019;73:1317-35. [Crossref] [PubMed]
- Liu X, Du T, Zhang H, Sun C. Detection and Classification of Chronic Total Occlusion lesions using Deep Learning. Annu Int Conf IEEE Eng Med Biol Soc 2019;2019:828-31. [Crossref] [PubMed]


