
Ewing’s sarcoma/primitive neuroectodermal tumor (ES/PNET) of the bladder in an adolescent: a case description
Introduction
Extraosseous Ewing’s sarcoma/primitive neuroectodermal tumors (ES/PNET) are a relatively rare group of malignant tumors (1,2) that occur in children and adolescents aged between 10–20 years (3). ES/PNET are highly aggressive and generally portend poor prognoses (4) because of the lack of clear clinical symptoms during the early stages. These tumors frequently occur in the extremities and retroperitoneum (5,6), and are seldom in the internal organs. ES/PNET of the bladder is rare, with only a few reports available at present (7,8). We report a case of primary ES of the bladder that occurred in a 19-year-old female.
Case presentation
A 19-year-old female patient was admitted to our hospital after experiencing gross hematuria without concomitant urinary frequency, urgency, painful urination, and blood clots for 1 week. Urine samples were routinely collected on admission, and the red blood cell count was 7,796/µL. The patient had been smoking for the previous year and had no family history of tumors.
Outpatient ultrasound suggested a left ureteral end cyst with abnormal internal echogenicity and clot but did not exclude the possibility of a tumor. Contrast-enhanced ultrasound (CEUS) suggested mixed (predominantly solid component) left end ureteral occupancy (Figure 1A). Computed tomography (CT) enhancement showed a cystic and solid mass in the lower left ureter, indicating the presence of tumor lesions. Magnetic resonance imaging (MRI) suggested that the left ureteral bladder wall was occupied, suggesting the possibility of a ureteral cyst combined with hemorrhage (Figure 1B,1C).
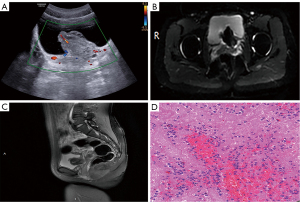
The patient then underwent cystoscopy and transurethral resection of bladder tumor (TURBT). Intraoperatively, the lesioned tissue had old hemorrhage and hematoma. Pathological examinations indicated microscopic infiltration and growth of small round tumor cells with higher nuclear plasma and darker nuclear chromatin (Figure 1D). Immunohistochemical staining of the paraffin tissue block A8 indicated that the lesion was positive for CD99, FLI1, Vimentin (+), SYN (focal weak), NSE (focal weak), β-catenin (cytosolic and cytoplasmic), INI-1, CD117, and Ki-67 (~40%), and negative for CgA, CK7, CK20, CK-pan, EMA, P63, GATA3, S100p, HCG, CD56, S100, Desmin, SMA, Myo-D1, Myogenin, and LCA. These findings were typical of extraosseous ES/PNET. The patient received gemcitabine infusion chemotherapy after surgery and was not subjected to further treatment. Notably, there was no sign of recurrence during the two years of follow-up.
All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committee(s), and the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
ES/PNET is a rare primary small round cell malignancy. It is difficult to establish clear guidelines for its management and treatment because of the minuscule number of reported cases. Its preoperative diagnosis is further complicated by the lack of specific imaging characteristics. Definitive diagnosis relies on postoperative pathology, immunohistochemistry, and genetic analysis. Early diagnosis and treatment of ES/PNET patients are thus highly challenging.
The case described in this report is of a 19-year-old female with clinical hematuria and hydronephrosis. The patient had a large lesion of approximately 55 mm ×36 mm, and had clear borders, irregular morphology, and convex growth towards the internal bladder. The lesion was close to the left ureteral bladder entrance, causing continuous compression to the left kidney and left ureteral fluid. The appearance of the lesion was very confusing on MRI, with significant low signals in T2WI and the center of DWI. The lesion had circumferential high signal at the edges, and T1WI showed equally high signal, slightly higher than the surrounding muscle signal. The internal signal of the lesion was more like a hematoma, and was also reflected in the surgical records. The pattern had a mild enhancement during the arterial phase, peaking during the venous phase, and decreasing during the delayed phase, with a net CT increase of approximately 8–29 HU. MRI showed discontinuous circumferential enhancement of the lesion with a significant visible gap in the right anterior.
In the previously reported cases (Table 1), the youngest patient was 10 years old, the oldest was 81 years old, bringing the mean age to 45 years. Notably, only one patient reported massive bleeding (25). Many patients had tumors on the right side of the bladder (7/21). In particular, there was extrinsic bladder protrusion towards the pelvis and the lesions were larger in most cases. However, the previous reports did not elaborate more on the imaging presentation. The imaging presentation of extraosseous Ewing sarcoma is not specific and may vary depending on the site. Generally, cystic necrosis is more common, calcification is rare, and pseudo-envelope may be present. Reinforcement varies depending on the site of occurrence and is mostly heterogeneous and variable (28,29). Ewing sarcoma of the bladder is rare but is more malignant and needs to be differentiated from the common uroepithelial carcinoma of the bladder. Based on the documented cases, Ewing sarcoma in the bladder seems to be common among middle-aged persons and young adults, while general uroepithelial carcinoma is common among middle-aged and older men. Notably, smoking is an important risk factor in both. CT enhancement demonstrates that there is progressive strengthening during uniform strengthening, with the most significant strengthening amplitude happening during the arterial phase (30). Of note, the final diagnosis of this disease relies on pathology because there are cases of ES combined with epithelial carcinoma. The imaging presentation of ES combined with epithelial carcinoma is somewhat different from that of most extraosseous ES because of the interference of massive bleeding. The descriptions of more new cases are thus necessary to improve the existing knowledge and experience.
Table 1
| References | Sex/age | Symptoms | Diagnostic | Tumor size (mm) | Local | Surgery |
|---|---|---|---|---|---|---|
| Present case | F/19 | Gross hematuria | US, CT, MRI | 55×36 | Left posterior | TURBT |
| Sueyoshi et al. (9) | M/10 | Polyuria, lower-abdominal swelling | US, CT | 135×131×129 | Right side | Double J tube + partial cystectomy |
| Osone et al. (10) | M/10 | Dysuria, hematuria, hematuria, fever | US, CT, cystoscope | 10 | Base | TURBT |
| Rao et al. (11) | F/14 | Dull pain, lower-abdominal lump | US, CT, needle biopsy | 150×120×75 | Posterior | Partial cystectomy |
| Gousse et al. (12) | F/15 | Hematuria | IVP, cystoscopy | 30×20×20 | Right anterior lateral | TURBT |
| Lopez-Beltran et al. (13) | F/21 | Frequency, dysuria, hematuria | US, cystoscope biopsy | 90×80×60 | The posterior and right and left sides | Cystectomy + TH + BSO |
| Banerjee et al. (14) | M/21 | Frequency, dysuria, hematuria | IVP, cystoscopy | 80×60×40 | Right side | Cystectomy |
| Vallonthaiel et al. (15) | F/27 | Frequency, hematuria | US, CT | 103×98×47 | Left anterior and lateral | TURBT |
| Lam et al. (16) | F/30 | Polyuria, hematuria | US, MRI | 64×94×77 | Right side | TURBT + cystectomy + Indiana pouch |
| Tonyalı et al. (17) | F/38 | Hematuria | CT | 40×26×25 | Right side | TURBT + cystectomy + TH + BSO + ileal conduit |
| Desai (18) | F/38 | Hematuria | Cystoscope | 120×70×35 | Posterior and right and left | Cystectomy + TH + BSO |
| Gao et al. (8) | F/45 | Frequency, urgency, dysuria | US, CT, cystoscope | 30 | Right neck | TURBT + cystectomy + TH + ileal conduit |
| Busato et al. (19) | F/52 | Frequency, dysuria, pelvic pain, hematuria | US, cystoscope | 33×15×22 | Right base | TURBT |
| Colecchia et al. (20) | F/61 | Hydronephrosis, renal failure | CT, cystoscope biopsy | – | – | – |
| Mentzel et al. (21) | M/62 | Dark urine, fever, backache, AUR | MRI | 140×100×100 | – | TURBT + nephrostomy |
| Liu et al. (7) | M/64 | Abdomen dull pain | CT | 60×50 | Left lateral out of bladder | – |
| Okada et al. (22) | M/65 | Hematuria, dysuria | US, CT, cystoscope | 50 | Left posterior | TURBT + cystectomy |
| Al Meshaan et al. (23) | F/67 | Hematuria, fever, hydronephrosis | US, CT, cystoscope | 30×25×10 | Posterior | TURBT + partial cystectomy |
| Ellinger et al. (24) | M/72 | Hematuria, oliguria | MRI | – | – | TURBT |
| Zheng et al. (25) | M/74 | Frequency, dysuria, hematuria | CT | – | Neck | TURBT |
| Zhang et al. (26) | F/78 | Gross hematuria, blood clot, urinary frequency, urgency | CTU | 63×44 | Right posterior | TURBT |
| Krüger et al. (27) | M/81 | Lymphedema, fatigue, urge, incontinence | US, CT | – | – | TURBT + nephrostomy |
ES/PNET, Ewing’s sarcoma/primitive neuroectodermal tumor; F, female; M, male; US, ultrasound; CT, computed tomography; MRI, magnetic resonance imaging; TURBT, transurethral resecting of bladder tumour; IVP, intravenous urography; TH, total hysterectomy; BSO, bilateral salpingo-oophorectomy; AUR, acute urinary retention; CTU, computed tomography urography.
Acknowledgments
We would like to thank MogoEdit (https://www.mogoedit.com) for its English editing during the preparation of this manuscript.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-867/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy and integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committee(s), and the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cabral GA, Nunes CF, Melo JO Jr, Guimarães RD, Gonçalves MB, Rodrigues RS, Correa JL, Teixeira OM Jr, Klescoski J Jr, Lapenta MA, Landeiro JA. Peripheral primitive neuroectodermal tumor of the cervical spine. Surg Neurol Int 2012;3:91. [Crossref] [PubMed]
- Dehner LP. Peripheral and central primitive neuroectodermal tumors. A nosologic concept seeking a consensus. Arch Pathol Lab Med 1986;110:997-1005. [PubMed]
- Scurr M, Judson I. How to treat the Ewing's family of sarcomas in adult patients. Oncologist 2006;11:65-72. [Crossref] [PubMed]
- Malek A. Md, Ziaee V Md, Kompani F Md, Moradinejad MH Md, Afzali N Md. Primitive neuroectodermal tumor, a rare cause of musculoskeletal manifestations in a child. Iran J Pediatr 2014;24:221-2. [PubMed]
- Martin RC 2nd, Brennan MF. Adult soft tissue Ewing sarcoma or primitive neuroectodermal tumors: predictors of survival? Arch Surg 2003;138:281-5. [Crossref] [PubMed]
- Kobayashi K, Tsutsumi S, Noguchi G, Osaka K, Umemoto S, Takeyama M, Hiruma T, Kishida T. A case of extraskeletal ewing's sarcoma in the retroperitoneum. Nihon Hinyokika Gakkai Zasshi 2020;111:89-93. [Crossref] [PubMed]
- Liu B, Qi DJ, Zhang QF. Primary adult primitive neuroectodermal tumor of the bladder: A case report and literature review. Medicine (Baltimore) 2020;99:e21740. [Crossref] [PubMed]
- Gao L, Xie W, Li K, Huang G, Ji Y, Ou Y, Chen J. Primitive neuroectodermal tumor of urinary bladder: A case report and literature review. Medicine (Baltimore) 2020;99:e23032. [Crossref] [PubMed]
- Sueyoshi R, Okawada M, Fujimura J, Saito M, Koga H, Lane GJ, Yamataka A. Successful complete resection of Ewing sarcoma arising from the bladder in a 10-year-old boy after chemotherapy. Pediatr Surg Int 2014;30:965-9. [Crossref] [PubMed]
- Osone S, Hosoi H, Tanaka K, Tsuchiya K, Iehara T, Morimoto A, Hashida T, Yamashita M, Kawabata K, Nishijo K, Toguchida J, Hata J, Sugimoto T. A case of a ewing sarcoma family tumor in the urinary bladder after treatment for acute lymphoblastic leukemia. J Pediatr Hematol Oncol 2007;29:841-4. [Crossref] [PubMed]
- Rao RN, Sinha S, Babu S, Mehrotra R. Fine-needle aspiration cytology of primitive neuroectodermal tumor of the urinary bladder: a case report. Diagn Cytopathol 2011;39:924-6. [Crossref] [PubMed]
- Gousse AE, Roth DR, Popek EJ, Cooley LD, Horowitz ME. Primary Ewing's sarcoma of the bladder associated with an elevated antinuclear antibody titer. J Urol 1997;158:2265-6. [Crossref] [PubMed]
- Lopez-Beltran A, Pérez-Seoane C, Montironi R, Hernández-Iglesias T, Mackintosh C, de Alava E. Primary primitive neuroectodermal tumour of the urinary bladder: a clinico-pathological study emphasising immunohistochemical, ultrastructural and molecular analyses. J Clin Pathol 2006;59:775-8. [Crossref] [PubMed]
- Banerjee SS, Eyden BP, McVey RJ, Bryden AA, Clarke NW. Primary peripheral primitive neuroectodermal tumour of urinary bladder. Histopathology 1997;30:486-90. [Crossref] [PubMed]
- Vallonthaiel AG, Kaur K, Jain D, Singh G, Tiwari D, Pramanik R, Singh P, Sharma MC. Ewing Sarcoma of Urinary Bladder Showing EWSR1 Rearrangement on FISH Analysis and Unique Response to Chemotherapy. Clin Genitourin Cancer 2016;14:e183-6. [Crossref] [PubMed]
- Lam CJ, Shayegan B. Complete resection of a primitive neuroectodermal tumour arising in the bladder of a 31-year-old female after neoadjuvant chemotherapy. Can Urol Assoc J 2016;10:E264-7. [Crossref] [PubMed]
- Tonyalı Ş, Yazıcı S, Yeşilırmak A, Ergen A. The Ewing's Sarcoma Family of Tumors of Urinary Bladder: A Case Report and Review of the Literature. Balkan Med J 2016;33:462-6. [Crossref] [PubMed]
- Desai S. Primary primitive neuroectodermal tumour of the urinary bladder. Histopathology 1998;32:477-8. [Crossref] [PubMed]
- Busato WF Jr, Almeida GL, Ogata DC. Primary primitive neuroectodermal tumor of the bladder: histologic and clinical features of 9 cases. Clin Genitourin Cancer 2011;9:63-7. [Crossref] [PubMed]
- Colecchia M, Dagrada GP, Poliani PL, Pilotti S. Immunophenotypic and genotypic analysis of a case of primary peripheral primitive neuroectodermal tumour (pPNET) of the urinary bladder. Histopathology 2002;40:108-9. [Crossref] [PubMed]
- Mentzel T, Flaschka J, Mentzel HJ, Eschholz G, Katenkamp D. Primary primitive neuroectodermal tumor of the urinary bladder. Clinicopathologic case report and differential small cell tumor diagnosis of this site. Pathologe 1998;19:154-8. [Crossref] [PubMed]
- Okada Y, Kamata S, Akashi T, Kurata M, Nakamura T, Kihara K. Primitive neuroectodermal tumor/Ewing's sarcoma of the urinary bladder: a case report and its molecular diagnosis. Int J Clin Oncol 2011;16:435-8. [Crossref] [PubMed]
- Al Meshaan MK, Nayef M, Kwaider T, Otto W, Katchy KC. Peripheral primitive neuroectodermal tumor of the urinary bladder in an Arab woman with history of squamous cell carcinoma: a case report. J Med Case Rep 2009;3:6840. [Crossref] [PubMed]
- Ellinger J, Bastian PJ, Hauser S, Biermann K, Müller SC. Primitive neuroectodermal tumor: rare, highly aggressive differential diagnosis in urologic malignancies. Urology 2006;68:257-62. [Crossref] [PubMed]
- Zheng Y, Tan F, Wang L, Xu N, Mou H. Primary primitive neuroectodermal tumor of the urinary bladder: a case report and literature review. Med Oncol 2011;28:S388-91. [Crossref] [PubMed]
- Zhang HZ, Wang SY, Shen XH. Ewing sarcoma of urinary bladder occurring simultaneously with high grade papillary urothelial carcinoma. Pathology 2020;52:612-5. [Crossref] [PubMed]
- Krüger S, Schmidt H, Kausch I, Böhle A, Holzhausen HJ, Johannisson R, Feller AC. Primitive neuroectodermal tumor (PNET) of the urinary bladder. Pathol Res Pract 2003;199:751-4. [Crossref] [PubMed]
- Somarouthu BS, Shinagare AB, Rosenthal MH, Tirumani H, Hornick JL, Ramaiya NH, Tirumani SH. Multimodality imaging features, metastatic pattern and clinical outcome in adult extraskeletal Ewing sarcoma: experience in 26 patients. Br J Radiol 2014;87:20140123. [Crossref] [PubMed]
- Javery O, Krajewski K, O'Regan K, Kis B, Giardino A, Jagannathan J, Ramaiya NH. A to Z of extraskeletal Ewing sarcoma family of tumors in adults: imaging features of primary disease, metastatic patterns, and treatment responses. AJR Am J Roentgenol 2011;197:W1015-22. [Crossref] [PubMed]
- Park S, Reuter VE, Hansel DE. Non-urothelial carcinomas of the bladder. Histopathology 2019;74:97-111. [Crossref] [PubMed]


