
Imaging appearances of paraganglioma of the urinary bladder
Introduction
Accounting for approximately 0.05% of all bladder tumors, paraganglioma of the urinary bladder (PUB) is a rare neuroendocrine tumor that originates from chromophores arising from neural crest tissue that vagally travels to the bladder wall during embryonic life (1). The most common clinical symptoms of PUB are hypertension, headache, hematuria, and palpitations, with characteristic micturition attacks. However, these symptoms are often nonspecific and uncommonly occur in one patient simultaneously, so the misdiagnosis rate is exceptionally high. In this report, we analyzed the imaging appearances of PUB confirmed by surgical resection and pathology.
Case presentation
Case 1
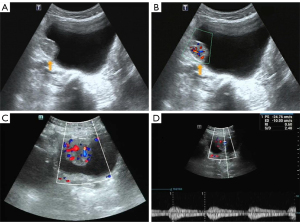
A 65-year-old man attended our hospital complaining of hematuria for 6 months. This patient had a history of hypertension and resection of a left lung squamous cell carcinoma 4 years ago. Ultrasound imaging (Aplio 500, Toshiba Medical Systems, Otawara, Japan; convex array probe; 1–6 MHz) showed a lesion (30 mm × 24 mm in size) located in the right bladder wall, which was regularly shaped and had a broad-based attachment to the bladder wall (Figure 1A). Color Doppler imaging also showed rich blood flow signals (Figure 1B).
Case 2
A 40-year-old woman presented with irregular menstruation for 4 months. Urine analysis showed microscopic hematuria 3+. Transabdominal gynecologic ultrasonography (Resona 7S, Mindray Medical Inc, Shenzhen, China; convex array probe with a frequency of 2–5 MHz) demonstrated a tumor in the right bladder wall, approximately 38 mm × 28 mm in size, with a regular shape and broad-base attachment to the bladder wall. Color Doppler imaging showed rich blood flow signals (Figure 1C), and the spectral Doppler exhibited a low resistance index (Figure 1D).
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). This study was approved by the ethics committee of The Second Affiliated Hospital of Nanchang University. Written informed consent was obtained from the patients for the publication of this case report and the accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
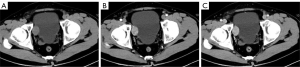
The lesion in case 1 showed isodensity (Figure 2A) on plain computed tomography (CT) scan (Siemens SOMATOM Definition Flash) as well as conspicuous enhancement (Figure 2B,2C). On magnetic resonance imaging (MRI; GE Signa HDxt 3.0T scanner), both lesions showed slightly higher intensity on T2-weighted imaging fat suppression (T2WI-FS), repetition time (TR): 2,560 ms; echo time (TE): 85 ms; Figure 3A) and T1-weighted imaging (T1WI; TR: 520 ms, TE: 7.6 ms; Figure 3B). Diffusion-weighted imaging (DWI; TR: 5,200 ms, TE: 68 ms) indicated strong hyperintensity (Figure 3C). Compared to ultrasound and CT, T2WI-FS clearly showed that the lesions were located in the submucosa of the bladder. After enhancement, the lesions showed conspicuous enhancement (Figure 3B,3D-3G).
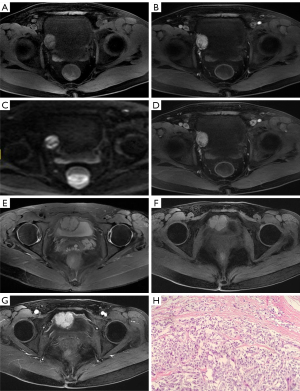
The patient in case 1 had received a cystoscopy in another hospital, which revealed that the tumor was oval and convex to the bladder cavity, with the surface of the tumor being rich in blood vessels. Considering that it might have been difficult to completely eradicate the tumor by transurethral resection of bladder tumor (TURBT), laparoscopic partial cystectomy was chosen. In case 2, the patient had elevated intraoperative blood pressure that reached up to 230/150 mmHg during the TURBT; thus, the operation was terminated and the resected tumor fragments were sent for pathological examination, which was confirmed to be PUB. Phenoxybenzamine was used to stabilize the patient’s blood pressure for days, and laparoscopic partial cystectomy was subsequently performed. Both cases’ postoperative pathology and immunohistochemistry confirmed the presence of PUB (Figure 3H). Ultrasound or CT was performed regularly in the 2 patients, and no signs of recurrence were found during the follow-up periods of 24 and 47 months, respectively.
Discussion
PUB was first reported by Zimmerman et al. in 1953. Beilan et al. (1) and Lee et al. (2) found that the incidence rates among males and females were similar and that PUB mainly occurred in young adults, mostly arising in the fourth or fifth decades of life. PUB is classified into functional or nonfunctional according to whether or not the tumor secretes catecholamines (3). The symptoms due to the hypersecretion of catecholamines can mimic more than 30 medical disorders (4). As for PUB, the major symptoms are hormonally active, and patients can present with clinical symptoms secondary to catecholamine release with micturition attacks such as hypertension, headache, hematuria, and palpitations. It is an extremely rare but severe condition that may cause malignant hypertension. However, nonfunctional PUB is rarer and more difficult to diagnose owing to its nonsecreting nature. Thus, the rate of PUB misdiagnosis is exceptionally high, especially among patients who complain of gross hematuria. Moreover, if the imaging examination shows a bladder lesion, misdiagnosis as bladder cancer is also common. It is known that 83% of PUBs exert endocrine functions (5), and tumor resection might stimulate the secretion of catecholamines, which may induce a hypertensive crisis. In cases where PUB is not considered, a lack of adequate treatment may threaten life; therefore, preoperative imaging examination plays a crucial role in localizing and characterizing the lesion.
PUB is an extra-adrenal tumor, which cannot be differentiated from pheochromocytoma based on histologic findings, so anatomical location is used to distinguish between them. Under a light microscope, PUB is located in the muscular layer of the bladder, and the tumor cells exhibit Zellballen growth that is practically the same irrespective of location. A single layer of flattened spindle-shaped supporting cells can be seen around the nest, and the interstitial vessels are abundant and sinus-like or lacunar-like, with a rich network of fibrous vessels between the nests.
PUB is the same as adrenal pheochromocytoma, which is a blood-rich tumor, and all cases imaged using contrast CT and MRI scanning exhibit conspicuous enhancement. The cytoplasm of chromophores is so rich in water and abundant in capillary networks and blood sinuses between the cell clusters that significant early enhancement will appear on CT and MRI (6). However, we found some different signs from those of previous reports of adrenal pheochromocytomas, such as necrosis and general cystic degeneration, but this phenomenon rarely occurs in PUB (5), which may be due to the rich vascular network of the interstitial substance. Unlike in adrenal pheochromocytoma, T2WI-FS has no typical “pepper salt” or “light-bulb” signs (7), which are more likely to appear in lesions of the head and neck (8). During the embryonic period, chromaffin tissue generated by the sympathetic plexus is present in the bladder muscle layer and is thought to be the source of these tumors. A bladder mucosal line can be seen around its periphery. On MRI, all of the lesions are located below the bladder mucosa; owing to its inherent tissue contrast resolution and multiple parametric imaging, MRI is superior to CT scan and ultrasound in terms of its diagnostic sensitivity and specificity for the submucosal origin of the tumor.
Other examinations also play an important role in its diagnosis. The biochemical tests for PUB mainly rely on the detection of blood and urine catecholamine levels, 24 h urine vanillic acid, and other signs. However, the level of catecholamines may not be increased in the resting stage of the tumor or in nonfunctional tumor. On cystoscopy, the tumor often appears as a single mass, with a wide base, and convex to the bladder cavity; moreover, the surface of the tumor is rich in blood vessels, and if hyperemia calcification, or necrosis of the bladder mucosa is indicated, it may be difficult to distinguish PUB from bladder cancer.
PUB is a rare bladder tumor that should be differentiated from other bladder occupancies, such as bladder cancer and bladder leiomyoma. Bladder cancer usually occurs in middle-aged and older patients, and intermittent and painless gross hematuria is the first symptom. Unlike that in PUB, the tumor in bladder cancer is located in the bladder mucosa, with a vegetable pattern, a nodular or irregular surface, few blood flow signals on color Doppler, and progressive enhancement on contrast CT or MRI scanning. Bladder leiomyoma is a benign tumor that resembles paraganglioma of the bladder in terms of its characteristics of a continuous bladder mucosal line. However, it is composed of fenestrated bundles of smooth muscle fibers arranged in a swirling pattern rather than in a nest-like structure. Furthermore, compared to PUB, bladder leiomyoma has an insufficient blood supply (9). Combining the abovementioned imaging features, clinical symptoms, laboratory examination, and cystoscopy can help to better distinguish between these conditions (10).
PUB exhibits a broad-based attachment to the bladder wall and displays the characteristic appearance of a bladder mucosal line around its periphery on ultrasound. MRI scanning can locate these tumors more clearly and precisely. Since PUB is a blood-rich tumor, color Doppler would show a significant blood flow signal, and contrast CT and MRI scanning would display conspicuous enhancement.
Conclusions
The various imaging appearances as presented on ultrasound, CT, and MRI can be complementary and display features that are useful for differentiating PUB from other bladder lesions. Moving forward, clinical symptoms and biochemical tests can also help make a more accurate preoperative diagnosis.
Acknowledgments
Funding: This work was supported by the Key Research and Development Plan of Jiangxi Province (No. 20202BBGL73032).
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-747/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki (as revised in 2013). The study was approved by the ethics committee of The Second Affiliated Hospital of Nanchang University.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Beilan JA, Lawton A, Hajdenberg J, Rosser CJ. Pheochromocytoma of the urinary bladder: a systematic review of the contemporary literature. BMC Urol 2013;13:22. [Crossref] [PubMed]
- Lee KY, Oh YW, Noh HJ, Lee YJ, Yong HS, Kang EY, Kim KA, Lee NJ. Extraadrenal paragangliomas of the body: imaging features. AJR Am J Roentgenol 2006;187:492-504. [Crossref] [PubMed]
- Zhai H, Ma X, Nie W, Li H, Peng C, Li X, Zhang Y, Zhang X. Paraganglioma of the Urinary Bladder: A Series of 22 Cases in a Single Center. Clin Genitourin Cancer 2017;15:e765-e771. [Crossref] [PubMed]
- Neumann HPH, Young WF Jr, Eng C. Pheochromocytoma and Paraganglioma. N Engl J Med 2019;381:552-65. [Crossref] [PubMed]
- Wang H, Ye H, Guo A, Wei Z, Zhang X, Zhong Y, Fan Z, Wang Y, Wang D. Bladder paraganglioma in adults: MR appearance in four patients. Eur J Radiol 2011;80:e217-20. [Crossref] [PubMed]
- Leung K, Stamm M, Raja A, Low G. Pheochromocytoma: the range of appearances on ultrasound, CT, MRI, and functional imaging. AJR Am J Roentgenol 2013;200:370-8. [Crossref] [PubMed]
- Zhang J, Bai X, Yuan J, Zhang X, Xu W, Ye H, Wang H. Bladder paraganglioma: CT and MR imaging characteristics in 16 patients. Radiol Oncol 2021;56:46-53. [Crossref] [PubMed]
- Itani M, Mhlanga J. Imaging of Pheochromocytoma and Paraganglioma. In: Mariani-Costantini R, editor. Paraganglioma: A Multidisciplinary Approach. Brisbane (AU): Codon Publications; 2019.
- Wu S. Imaging findings of atypical leiomyoma of the urinary bladder simulating bladder cancer: a case report and literature review. Med Ultrason 2013;15:161-3. [Crossref] [PubMed]
- Asa SL, Ezzat S, Mete O. The Diagnosis and Clinical Significance of Paragangliomas in Unusual Locations. J Clin Med 2018;7:280. [Crossref] [PubMed]


