
The value of 18F-fluorodeoxyglucose positron emission tomography/magnetic resonance whole-body scans and local enhancement scans in the preoperative staging and resectability assessment of pancreatic adenocarcinoma
Introduction
In recent years, the global incidence of pancreatic cancer has been increasing. It is recognized as a highly malignant tumor, ranking fourth in the United States (1) and sixth in China (2) in terms of malignant tumor-related mortality. In addition, the early diagnosis of pancreatic ductal adenocarcinoma is difficult, and surgical resection is still the only way to potentially cure it. Nevertheless, more than half of patients have missed the opportunity for radical surgery when they first seek medical advice; hence, it is essential for patients to have a precise assessment before surgery. According to imaging results, nonmetastatic tumors are divided into 3 categories to personalize therapeutic decision-making: resectable, borderline resectable, and unresectable. Patients with borderline resectable tumors may have the opportunity for radical surgery with a successful downstaging of disease through neoadjuvant therapy (3,4). Comprehensive multidisciplinary treatment is the preferred therapeutic schedule for patients with locally advanced disease and distant metastases, and accurate preoperative assessment is particularly significant when treating pancreatic ductal adenocarcinoma. Dynamic contrast-enhanced multiphase thin slice computed tomography (CT) or magnetic resonance imaging (MRI) is the standard imaging examination for the initial diagnosis and preoperative staging of pancreatic cancer. For patients with high-risk tumors, 18F-flurodeoxyglucose (18F-FDG) positron emission tomography (PET)/CT is recommended (3). It is difficult to detect isodense pancreatic cancer and differentiate some small hepatic lesions with CT, while MRI can perform a supplementary function (5,6). Furthermore, CT and MRI play a limited role in restaging pancreatic cancer after treatment (7). 18F-FDG PET/CT demonstrates obvious superiority in detecting extrapancreatic metastases and evaluating tumor burden but has a limited role in primary tumor staging. 18F-FDG PET/magnetic resonance (MR) has the combined advantages of whole-body examination with 18F-FDG PET and high soft-tissue resolution with MRI; hence, it has potential value in malignant tumor staging (8-10). 18F-FDG PET/MR has not been used in clinical practice for very long; therefore, its value in the preoperative evaluation of pancreatic cancer has not been fully assessed (11). This study aimed to explore the value of a preoperative assessment using hybrid 18F-FDG PET/MR whole-body imaging of pancreatic cancer patients with surgical intent. We present the following article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-731/rc).
Methods
Patients
This is a retrospective cohort study. Data collection (of imaging, clinical, and pathological information) was conducted from April 2022 to June 2022 from patients who underwent 18F-FDG PET/MR with clinically diagnosed or suspected pancreatic cancer from March 2018 to March 2022 at Ruijin Hospital. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Institutional Ethics Committee of Ruijin Hospital approved this study, and individual consent for this retrospective analysis was waived. The following inclusion criteria were applied: (I) patients with either clinically diagnosed or suspected pancreatic cancer according to available clinical information or imaging examination and (II) patients who had undergone integrated whole-body 18F-FDG PET MR imaging before surgery.
The exclusion criteria for the analysis were the following: (I) patients with no definite pathological diagnosis obtained within 30 days after 18F-FDG PET/MR examination (16 cases), (II) patients with pathological diagnosis or clinical consideration of nonpancreatic ductal adenocarcinoma (PDAC) disease (14 cases), and (III) patients with a previous diagnosis of other malignant tumors (1 case).
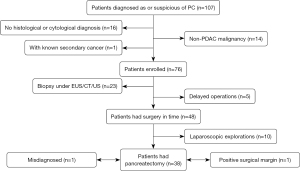
From March 2018 to March 2022, 107 patients conformed to the inclusion criteria, while 31 patients were excluded from the study based on the exclusion criteria. Among those excluded, 16 patients did not obtain a definite pathological diagnosis within 30 days after the 18F-FDG PET/MR examination, 14 patients were diagnosed with non-PDAC disease (including 1 duodenal papillary carcinoma, 1 duodenal adenocarcinoma, 1 squamous cell carcinoma, 1 non-Hodgkin lymphoma, 2 small cell neuroendocrine carcinomas, 7 pancreatic neuroendocrine tumors, and 1 immunoglobin G4–related autoimmune pancreatitis), and 1 patient was diagnosed with thyroid cancer before being diagnosed with pancreatic cancer (Figure 1). The included patients were followed up through outpatient or inpatient visits and by regular imaging appointments from May 2018 to May 2022.
PET/MR protocol
All 18F-FDG PET/MR imaging was performed on a Biograph mMR scanner (Siemens Healthineers, Erlangen, Germany). Patients fasted for at least 6 hours before the examination to control their blood glucose which was checked with a fingertip stick before administration of 18F-FDG via injection. The injection dose of 18F-FDG was 5.6±1.1 mCi (3.5–9.4 mCi). Integrated PET/MR imaging was performed in 5 bed positions (acquisition time, 4 min/bed position) with a 60–90 min delay between injection and scanning. In 59 patients, an additional local abdominal scan was performed after whole-body scanning (1 bed position, 15 min/bed position).
A simultaneous, whole-body MRI was acquired, including transverse T2-weighed imaging (T2WI) using half-Fourier acquisition single-shot turbo spin-echo (HASTE) sequences, T1-weighted imaging (T1WI) using Dixon-type (in-phase, out-of-phase, water-only phase, fat-only phase) sequences, diffusion-weighted imaging (DWI), and apparent diffusion coefficient (ADC)–calculated imaging (b=50,800). T2WI with water-suppression and dark-fluid turbo spin-echo (TSE) scanning was also performed on the heads of the patients. Additional local abdominal MR scanning was performed, including transverse T2WI with fat suppression (T2WI-fs), DWI and ADC-calculated imaging (b=50,800), coronal T2WI-fs and T2W HASTE imaging, transverse T1WI with fat suppression (T1WI-fs) volumetric interpolated breath-hold examination (VIBE) imaging conducted before and after dynamic contrast-enhanced (DCE) imaging, and coronal T1WI-fs with contrast-enhanced imaging. Transverse contrast-enhanced T1WI-fs VIBE images were acquired throughout the body, and transverse T1WI-fs TSE was obtained in the brain. Among all of the patients, 62 underwent a dynamic contrast-enhanced MR (DCEMR) scan. Further details regarding the PET and MRI imaging acquisition parameters are displayed in the supplementary materials (Tables S1,S2).
Image interpretation
The PET/MR image analysis was performed on a professional workstation (Syngo.via; Siemens Healthineers). The diagnosis and staging were jointly completed on the PET/MR images by a junior nuclear medicine doctor, a senior nuclear medicine doctor, and a senior radiologist. The stage of pancreatic cancer was based on the American Joint Committee on Cancer (AJCC) tumor–node–metastasis (TNM) staging system (eighth edition) and the 2021 National Comprehensive Cancer Network (NCCN) guidelines (3). The 18F-FDG PET/MR staging methodology for primary pancreatic cancer was applied as follows: the maximum diameter of a lesion was measured on the MRI images. The first choice of images was T1WI-fs images in the pancreatic parenchymal phase, supplemented by contrast-enhanced coronal T1WI-fs or T2WI-fs images. Cases without enhanced scanning were measured on transverse T1WI-fs images, and lesions with isointensity on T1WI were measured on T2WI images. ADC measurements were applied to the largest levels of each lesion, with blood vessels and cystic areas being avoided. Metabolic parameters were measured on whole-body PET images. The metabolic tumor volume (MTV) calculation was based on 40% of the maximum standardized uptake value (SUVmax) of the lesion as the threshold to automatically extract the volume of interest. The total lesion glycolysis (TLG) was calculated as the product of the MTV multiplied by the SUVmean. With PET/MR, the following diagnostic criteria for positive lymph nodes were applied: increased 18F-FDG uptake in lymph nodes (SUVmax >2.5), enlarged lymph nodes (short diameter >0.8 cm), or lymph nodes with inhomogeneous enhancement. With PET/MR, the following diagnostic criteria for a distant metastasis were applied: hypermetabolic lesions in PET images with abnormal signals in MR images; however, these did not meet the diagnosis of well-known benign lesions. The clinical TNM (cTNM) staging and assessment of the resectability of pancreatic cancer of the patients were evaluated according to the 18F-FDG PET/MR findings.
The data relevant to this study were gathered by interrogating the hospital information system for patient demographic characteristics, serum tumor markers, clinical diagnoses and treatment plans before 18F-FDG PET/MR, multidisciplinary team (MDT) discussions and recommendations, staging after 18F-FDG PET/MR, therapeutic strategy, operation dates, intraoperative findings, and pathological diagnoses. The criteria for resectability in our center are based on the NCCN guidelines 2021 (3). Decisions about resectability status were made in consensus at multidisciplinary discussions. A patient was defined as resectable when there was no arterial tumor contact [on the celiac axis (CA), superior mesenteric artery (SMA), or common hepatic artery (CHA)] and no tumor contact with the superior mesenteric vein (SMV) or portal vein (PV), or ≤180° tumor contact without vein contour irregularity. The patient was defined as unresectable when there was solid tumor contact of >180° with the SMA or CA, aortic involvement, or unreconstructible SMV or PV due to tumor involvement or occlusion (by tumor or bland thrombus). Patients who did not meet either resectable or unresectable criteria were defined as borderline resectable according to the 2021 NCCN guidelines (3). A stage T4 tumor was diagnosed according to the considerations of the preoperative MDT, intraoperative findings of vascular invasion, and histopathological results. The pathological results of lesions outside the operation area were obtained by a biopsy to meet the needs of staging and treatment decisions. Patients with distant metastatic lesions without a pathological diagnosis were confirmed by other imaging examinations at the same period or by follow-up imaging performed within 3 months. The accuracy of 18F-FDG PET/MR in staging pancreatic cancer and assessing surgical resectability was evaluated based on the pathological results, intraoperative findings, and documented final clinical stages of illness. The adjustment of patient treatment plans was also analyzed before and after 18F-FDG PET/MR examinations.
Statistical analysis
All analyses were carried out using the SPSS v. 23 software (IBM Corp., Armonk, NY, USA). The quantitative parameters which conformed to the normal distribution are expressed by mean ± standard deviation, and the others are described by median (interquartile range). The qualitative characteristics are described by count and percentage. The difference of the normally distributed quantitative parameters was compared by the independent samples t-test, while others were compared by the Mann-Whitney test. The qualitative characteristics were compared by the chi-squared test and Fisher exact test. For all statistical tests, a 2-tailed P value <0.05 was considered to be statistically significant.
Results
A total of 47 males and 29 females were finally included in this study, with an average age of 61±10 years (range, 32–84 years). A summary of the clinical and imaging characteristics of the 76 pancreatic cancer patients is displayed in Table 1. Of the 76 patients, 53 had undergone surgery, and 5 of these patients were excluded from the evaluation of resectability and pathological staging because the period of time between their operation and the PET/MR examination was more than 30 days. For the other 48 patients, the average interval between the PET/MR examination and surgery was 4 days (range, 3–7 days). Of these 48 patients, 38 underwent pancreatic tumor resection (including 1 patient with a positive margin) and 10 (9 patients with distant metastasis and 1 patient with locally advanced diseases) underwent laparoscopic exploration. Among the 23 patients who did not have an operation, 16 cases had distant metastasis and 7 cases had locally advanced diseases (Figure 1).
Table 1
| Feature | All 76 cases | Clinical stage (I–II, 32 cases) | Clinical stage (III–IV, 44 cases) | P value (statistical value)* |
|---|---|---|---|---|
| Age (year), mean ± SD (range) | 61±10 (32–84) | 63±12 | 60±9 | 0.158 (t=1.426) |
| Gender, male/female | 47/29 | 23/9 | 24/20 | 0.343 (χ2=3.446) |
| Body weight (kg), median (IQR) | 60 (69–55) | 63 | 60 | 0.397 (U=623.5) |
| BMI, mean ± SD (range) | 22.4±3.3 (15.2–30.8) | 22.5±3.4 | 22.2±3.2 | 0.698 (t=0.390) |
| Blood glucose (mmol/L), median (IQR) | 6.4 (7.6–5.9) | 6.4 | 6.5 | 0.490 (U=638.5) |
| CA19–9 (U/mL), median (IQR) | 104.7 (413.4–22.9) | 74.5 | 158.7 | 0.256 (U=582.0) |
| CA125 (U/mL), median (IQR) | 16.15 (39.0–8.5) | 14.4 | 23.5 | 0.051 (U=493.5) |
| CEA (ng/mL), median (IQR) | 3.20 (6.5–1.80) | 2.27 | 3.66 | 0.154 (U=541.5) |
| Location | 0.030 (χ2=12.423) | |||
| Head, n (%) | 29 (38.2) | 16 (21.1) | 13 (17.1) | |
| Body and tail, n (%) | 41 (53.9) | 14 (18.4) | 26 (34.2) | |
| Multiple, n (%) | 6 (7.9) | 1 (1.3) | 5 (6.6) | |
| Size (cm), median (IQR) | 3.5 (4.7–2.9) | 3.2 | 3.9 | 0.002 (U=394.0) |
| SUVmax, median (IQR) | 6.6 (9.3–4.6) | 5.0 | 7.5 | 0.039 (U=507.5) |
| SUVpeak, median (IQR) | 5.1 (6.9–3.5) | 4.2 | 5.6 | 0.008 (U=453.0) |
| SUVmean, median (IQR) | 3.7 (5.1–2.6) | 2.9 | 4.4 | 0.049 (U=517.0) |
| MTV (cm3), median (IQR) | 12.9 (25.5–7.7) | 8.0 | 17.0 | 0.002 (U=4 14.0) |
| TLG, median (IQR) | 64.9 (88.5–26.1) | 28.4 | 75.9 | <0.001 (U=344.0) |
| ADCmin (103 mm2/s), mean ± SD | 1.02±0.23 | 1.08±0.22 | 0.99±0.24 | 0.104 (t=1.467) |
| ADC mean (103 mm2/s), mean ± SD | 1.33±0.23 | 1.36±0.23 | 1.32±0.24 | 0.470 (t=0.726) |
*, comparisons between clinical stage I–II versus III–IV. IQR, interquartile range; BMI, body mass index; SD, stand deviation; CA19–9, cancer antigen 19–9; CA125, cancer antigen 125; CEA, carcinoembryonic antigen; SUV, standardized uptake value; max, maximum; MTV, metabolic tumor volume; TLG, total lesion glycolysis; ADC, apparent diffusion coefficient; min, minimum; U, Mann-Whitney U test.
One of the 38 patients with pancreatectomy was misdiagnosed as having inflammatory disease by preoperative PET/MR, and the tumor staging and resectability were not evaluated in the PET/MR report. The pathological diagnosis was, in fact, pancreatic colloid carcinoma after radical subtotal pancreatectomy. Based on the results of pathology and final clinical staging, the accuracy of 18F-FDG PET/MR for cTNM staging (stages I, II, III, and IV) of pancreatic cancer was 73.3% (55/75; Table 2). The area under the curve (AUC) of 18F-FDG PET/MR for diagnosing advanced stage (III–IV) versus nonadvanced stage (I–II) pancreatic cancer was 0.922 [95% confidence interval (CI): 0.852–0.993] while the accuracy for diagnosing a distant metastasis of pancreatic cancer was 94.7% (71/75). Patients in the advanced stages of disease had the following characteristics compared to those in the nonadvanced stages: lesions in the pancreatic body and tail or involving multiple sections of the pancreas (P=0.030), larger tumor (P=0.002), higher SUVs (including SUVmax, P=0.039; SUVpeak, P=0.008; and SUVmean, P=0.049), higher MTV (P=0.002), and higher TLG (P<0.001; Table 1). There were no significant statistical differences in the following characteristics between advanced patients and nonadvanced patients: sex (P=0.343), age (P=0.158), weight (P=0.397), BMI (P=0.698), blood glucose (P=0.490), cancer antigen (CA) 19–9 (P=0.256), CA 125 (P=0.051), carcinoembryonic antigen (CEA; P=0.154), and ADC values (ADCmin, P=0.104; ADCmean, P=0.470) of the lesions (Table 1).
Table 2
| Preoperative 18F-FDG PET/MR stage | Clinical stage | |||
|---|---|---|---|---|
| I | II | III | IV | |
| I | 12 | 13 | 3 | 0 |
| II | 0 | 4 | 1 | 0 |
| III | 0 | 2 | 12 | 1 |
| IV | 0 | 0 | 0 | 27 |
18F-FDG PET/MR, 18F-fluorodeoxyglucose positron emission tomography/magnetic resonance.
The treatment regimen of 20.0% (15/75) of patients was impacted by 18F-FDG PET/MR. Among the 15 patients, 8 switched from surgery to neoadjuvant chemotherapy because 7 were diagnosed with locally advanced stage of disease and 1 was diagnosed with borderline resectable disease at preoperative assessment. Of the 8 patients mentioned above, 2 received radical surgery and neoadjuvant therapy following their tumor downstaging (Figures 2,3). Of the 15 patients mentioned above, 5 had distant metastasis, 1 had borderline resectable pancreatic cancer, and all 6 of these patients underwent laparoscopic exploration and biopsy. One patient achieved tumor downstaging after neoadjuvant chemotherapy and underwent local treatment of the single liver metastasis followed by radical surgery.
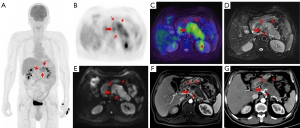
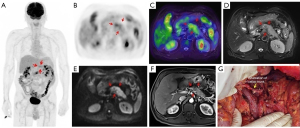
The accuracy of 18F-FDG PET/MR in evaluating the resectability of pancreatic cancer was 91.9% (34/37; Table 3). There were 14 cases of borderline resectable tumors in this study, 2 of whom were judged to be resectable before the operation. Of these 2 cases, 1 underwent hepatic artery suture and the other underwent partial resection of the PV during the operation. There were 21 cases of resectable tumors. One case was considered borderline resectable before the operation and underwent radical distal pancreatectomy. Preoperative PET/MR showed tumor tissue contact with the left side of the celiac trunk. The preoperative judgment of the 2 cases with locally advanced tumors was consistent with the intraoperative findings. With the surgical and pathological results taken as a reference, the overall accuracy of preoperative 18F-FDG PET/MR in T staging (T1, T2, T3, T4) was 62.2%, the AUC for diagnosing T4 and T1–3 stages were 0.872 (95% CI: 0.660–1.000), and the accuracy for N staging (N0 and N1–2) was 56.8% (21/37; Table 4).
Table 3
| Preoperative 18F-FDG PET/MR assessment | Surgical/pathological result | Sum | ||
|---|---|---|---|---|
| Resectable | Borderline resectable | Locally advanced | ||
| Resectable | 20 | 2 | 0 | 22 |
| Borderline resectable | 1 | 12 | 0 | 13 |
| Locally advanced | 0 | 0 | 2 | 2 |
| Sum | 21 | 14 | 2 | |
18F-FDG PET/MR, 18F-fluorodeoxyglucose positron emission tomography/magnetic resonance.
Table 4
| Preoperative 18F-FDG PET/MR assessment | Pathological result | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary tumor stage | Regional lymph node | Stage | |||||||||||||
| T1 | T2 | T3 | T4 | Sum | N0 | N1–2 | Sum | I | II | III | IV | Sum | |||
| T1 | 3 | 4 | 0 | 0 | 7 | ||||||||||
| T2 | 3 | 12 | 4 | 0 | 19 | ||||||||||
| T3 | 0 | 2 | 5 | 0 | 7 | ||||||||||
| T4 | 0 | 1 | 0 | 3 | 4 | ||||||||||
| Sum | 6 | 19 | 9 | 3 | |||||||||||
| N0 | 17 | 12 | 29 | ||||||||||||
| N1-2 | 0 | 4 | 4 | ||||||||||||
| Uncertain | 1 | 3 | 4 | ||||||||||||
| Sum | 18 | 19 | – | ||||||||||||
| Stage | |||||||||||||||
| I | 12 | 13 | 1 | 0 | 26 | ||||||||||
| II | 0 | 3 | 1 | 0 | 4 | ||||||||||
| III | 0 | 1 | 3 | 0 | 4 | ||||||||||
| IV | 0 | 0 | 0 | 3 | 3 | ||||||||||
| Sum | 12 | 17 | 5 | 3 | |||||||||||
18F-FDG PET/MR, 18F-fluorodeoxyglucose positron emission tomography/magnetic resonance; T, primary tumor; N0, no regional lymph node metastasis; N1–2, metastasis in the regional lymph node.
Discussion
This study found that the performance of 18F-FDG PET/MR in the overall staging of a primary tumor (T1, T2, T3, and T4) and the AJCC prognostic groups (I, II, III, IV) was poor. The accuracy of 18F-FDG PET/MR in distinguishing between resectable and nonresectable pancreatic cancer (including locally advanced tumor and distant metastasis) was high. The 18F-FDG PET/MR features of pancreatic cancer showed significant differences between stages III–IV and I–II, with the former having larger lesions and higher FDG uptake. The results proved that 18F-FDG PET could reveal the metabolic activity and burden of the tumor. 18F-FDG PET has advantages in detecting or excluding distant metastatic lesions, so it is recommended in clinical practice for patients with large primary lesions, suspected regional lymphadenopathy, and significantly high CA19–9 levels (3). The results of our study are similar to those of previous studies (12,13), in that higher stages of pancreatic cancer tended to have higher MTV and TLG values and thus a higher tumor burden and poorer prognosis. 18F-FDG PET/MR examination may show extrapancreatic metastases that cannot be discovered by conventional CT or MRI (14), and this information can be used to inform patient’s treatment plans, accordingly (e.g., by avoiding open surgery). For patients who cannot be operated on but undergo radiotherapy and chemotherapy, the effect of treatment can be monitored early (e.g., through changes in glucose metabolism) to provide information to support the timely adjustment of therapeutic options and the selection of more effective treatment methods (15,16). About one-fifth of the patients in this study had their initial treatment scheme adjusted after 18F-FDG PET/MR examination, and this result was similar to those reported in previous studies (11). Furtado et al. (17) even reported that a higher proportion (49%) of patients changed their clinical treatment plans after PET/MRI, which was higher than that for other routine imaging examinations in the same period. The reason behind this difference in results could be the difference in the proportion of stage III–IV patients in the studies; the previous study had a higher proportion than our study (86.5% vs. 58.7%, respectively).
In this study, the accuracy of 18F-FDG PET/MR for the resectability of pancreatic cancer was 91.9%, which is close to that in previous studies (18). Studies have shown that surgeons have high consistency in the judgment of locally advanced disease but relatively low consistency for the borderline resectable status, which can be affected by subjective factors (19).
18F-FDG PET/MR has a low sensitivity to regional lymph node metastasis of pancreatic cancer, a considerable number of metastatic lymph nodes being peritumor lymph nodes. These lymph nodes are usually not obviously enlarged; therefore, it is difficult to determine whether they are metastatic or not with preoperative imaging. The diagnostic accuracy for this study’s N stage (N0 and N1-2) was 56.8%, which is similar to previous studies (18,20). We also found that stage II and stage III are easily underestimated. The overall accuracy for AJCC prognostic groups consequently decreased due to the low sensitivity of N staging.
There are some limitations to our study. First, PET/MR has limitations in diagnosing of atypical pancreatic adenocarcinoma, such as colloid carcinoma. The lesion of colloid carcinoma is mainly composed of extracellular mucin, with low FDG metabolism and low enhancement. In this study, 1 of 76 pancreatic patients with adenocarcinoma was misdiagnosed as having inflammatory disease. Even though 18F-FDG PET/MR has a high diagnostic accuracy in the clinical staging of PDAC, the probability of not diagnosing PDAC is about 1.3%. Second, this study employed a single-center, retrospective design with a small number of cases. We expect our results can be confirmed in large-sample, prospective studies in the future. Third, some patients with advanced tumors, mainly stage IV patients with distant metastasis, did not undergo local scanning or DCE scanning. Finally, not all distant metastases were diagnosed pathologically; other imaging examinations in the same period or follow-up data were available as the reference standard.
Conclusions
18F-FDG PET/MR performs well in diagnosing advanced pancreatic cancer and impacts the treatment decisions of a considerable number of patients. 18F-FDG PET/MR has high accuracy in evaluating the resectability of pancreatic cancer before surgery and can provide comprehensive information for high-risk patients, helping to make a personalized treatment plan.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (No. 62073218) and the Science and Technology Committee of Shanghai Municipality (No. 20Y11912300).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-731/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-731/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Institutional Ethics Committee of Ruijin Hospital approved this study, and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- Zheng R, Zhang S, Zeng H, Wang S, Sun K, Chen R, Li L, Wei W, He J. Cancer incidence and mortality in China, 2016. Journal of the National Cancer Center 2022;2:1. [Crossref]
- Tempero MA, Malafa MP, Al-Hawary M, Behrman SW, Benson AB, Cardin DB, et al. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2021;19:439-57. [Crossref] [PubMed]
- Heinrich S, Besselink M, Moehler M, van Laethem JL, Ducreux M, Grimminger P, Mittler J, Lang H, Lutz MP, Lesurtel M. Scientific and Research Committee of the E-AHPBA and the EORTC pancreas working group. Opinions and use of neoadjuvant therapy for resectable, borderline resectable, and locally advanced pancreatic cancer: international survey and case-vignette study. BMC Cancer 2019;19:675. [Crossref] [PubMed]
- Zhang Z, Jia G, Pan G, Cao K, Yang Q, Meng H, Yang J, Zhang L, Wang T, Cheng C, Zuo C. Comparison of the diagnostic efficacy of (68) Ga-FAPI-04 PET/MR and (18)F-FDG PET/CT in patients with pancreatic cancer. Eur J Nucl Med Mol Imaging 2022;49:2877-88. [Crossref] [PubMed]
- Kim HJ, Park MS, Lee JY, Han K, Chung YE, Choi JY, Kim MJ, Kang CM. Incremental Role of Pancreatic Magnetic Resonance Imaging after Staging Computed Tomography to Evaluate Patients with Pancreatic Ductal Adenocarcinoma. Cancer Res Treat 2019;51:24-33. [Crossref] [PubMed]
- Wagner M, Antunes C, Pietrasz D, Cassinotto C, Zappa M, Sa Cunha A, Lucidarme O, Bachet JB. CT evaluation after neoadjuvant FOLFIRINOX chemotherapy for borderline and locally advanced pancreatic adenocarcinoma. Eur Radiol 2017;27:3104-16. [Crossref] [PubMed]
- Hu S, Xing X, Liu J, Liu X, Li J, Jin W, Li S, Yan Y, Teng D, Liu B, Wang Y, Xu B, Du X. Correlation between apparent diffusion coefficient and tumor-stroma ratio in hybrid (18)F-FDG PET/MRI: preliminary results of a rectal cancer cohort study. Quant Imaging Med Surg 2022;12:4213-25. [Crossref] [PubMed]
- Martin O, Schaarschmidt BM, Kirchner J, Suntharalingam S, Grueneisen J, Demircioglu A, Heusch P, Quick HH, Forsting M, Antoch G, Herrmann K, Umutlu L. PET/MRI Versus PET/CT for Whole-Body Staging: Results from a Single-Center Observational Study on 1,003 Sequential Examinations. J Nucl Med 2020;61:1131-6. [Crossref] [PubMed]
- Beiderwellen K, Grueneisen J, Ruhlmann V, Buderath P, Aktas B, Heusch P, Kraff O, Forsting M, Lauenstein TC, Umutlu L. [(18)F]FDG PET/MRI vs. PET/CT for whole-body staging in patients with recurrent malignancies of the female pelvis: initial results. Eur J Nucl Med Mol Imaging 2015;42:56-65. [Crossref] [PubMed]
- Lee JW. O JH, Choi M, Choi JY. Impact of F-18 Fluorodeoxyglucose PET/CT and PET/MRI on Initial Staging and Changes in Management of Pancreatic Ductal Adenocarcinoma: A Systemic Review and Meta-Analysis. Diagnostics (Basel) 2020;10:952. [Crossref] [PubMed]
- Chen BB, Tien YW, Chang MC, Cheng MF, Chang YT, Wu CH, Chen XJ, Kuo TC, Yang SH, Shih IL, Lai HS, Shih TT. PET/MRI in pancreatic and periampullary cancer: correlating diffusion-weighted imaging, MR spectroscopy and glucose metabolic activity with clinical stage and prognosis. Eur J Nucl Med Mol Imaging 2016;43:1753-64. [Crossref] [PubMed]
- Gao J, Huang X, Meng H, Zhang M, Zhang X, Lin X, Li B. Performance of Multiparametric Functional Imaging and Texture Analysis in Predicting Synchronous Metastatic Disease in Pancreatic Ductal Adenocarcinoma Patients by Hybrid PET/MR: Initial Experience. Front Oncol 2020;10:198. [Crossref] [PubMed]
- Zhang Z, Zhou N, Guo X, Li N, Zhu H, Yang Z. Pretherapeutic Assessment of Pancreatic Cancer: Comparison of FDG PET/CT Plus Delayed PET/MR and Contrast-Enhanced CT/MR. Front Oncol 2022;11:790462. [Crossref] [PubMed]
- Evangelista L, Zucchetta P, Moletta L, Serafini S, Cassarino G, Pegoraro N, Bergamo F, Sperti C, Cecchin D. The role of FDG PET/CT or PET/MRI in assessing response to neoadjuvant therapy for patients with borderline or resectable pancreatic cancer: a systematic literature review. Ann Nucl Med 2021;35:767-76. [Crossref] [PubMed]
- Harder FN, Jungmann F, Kaissis GA, Lohöfer FK, Ziegelmayer S, Havel D, Quante M, Reichert M, Schmid RM, Demir IE, Friess H, Wildgruber M, Siveke J, Muckenhuber A, Steiger K, Weichert W, Rauscher I, Eiber M, Makowski MR, Braren RF. [18F]FDG PET/MRI enables early chemotherapy response prediction in pancreatic ductal adenocarcinoma. EJNMMI Res 2021;11:70. [Crossref] [PubMed]
- Furtado FS, Ferrone CR, Lee SI, Vangel M, Rosman DA, Weekes C, Qadan M, Fernandez-Del Castillo C, Ryan DP, Blaszkowsky LS, Hong TS, Clark JW, Striar R, Groshar D, Cañamaque LG, Umutlu L, Catalano OA. Impact of PET/MRI in the Treatment of Pancreatic Adenocarcinoma: a Retrospective Cohort Study. Mol Imaging Biol 2021;23:456-66. [Crossref] [PubMed]
- Joo I, Lee JM, Lee DH, Lee ES, Paeng JC, Lee SJ, Jang JY, Kim SW, Ryu JK, Lee KB. Preoperative Assessment of Pancreatic Cancer with FDG PET/MR Imaging versus FDG PET/CT Plus Contrast-enhanced Multidetector CT: A Prospective Preliminary Study. Radiology 2017;282:149-59. [Crossref] [PubMed]
- Wittel UA, Lubgan D, Ghadimi M, Belyaev O, Uhl W, Bechstein WO, Grützmann R, Hohenberger WM, Schmid A, Jacobasch L, Croner RS, Reinacher-Schick A, Hopt UT, Pirkl A, Oettle H, Fietkau R, Golcher H. Consensus in determining the resectability of locally progressed pancreatic ductal adenocarcinoma - results of the Conko-007 multicenter trial. BMC Cancer 2019;19:979. [Crossref] [PubMed]
- Asagi A, Ohta K, Nasu J, Tanada M, Nadano S, Nishimura R, Teramoto N, Yamamoto K, Inoue T, Iguchi H. Utility of contrast-enhanced FDG-PET/CT in the clinical management of pancreatic cancer: impact on diagnosis, staging, evaluation of treatment response, and detection of recurrence. Pancreas 2013;42:11-9. [Crossref] [PubMed]


