
Efficacy and safety of high-intensity focused ultrasound ablation for rectus abdominis endometriosis: a 7-year follow-up clinical study
Introduction
In vivo, high-intensity focused ultrasound (HIFU) causes protein degeneration in the treatment area and no damage to surrounding tissue via focused ultrasonic waves (1-3), as the focused tissue temperature rises to between 65 ℃ and 100 ℃ instantaneously. Although anesthesia is not required, sedation is widely recommended during treatment (1,2). Moreover, although the safety and efficacy of HIFU have been established in long-term clinical follow-up studies following the ablation of uterine fibroids (1), abdominal wall endometriosis (AWE) (2,3), and solid tumors (4), there are currently no clinical follow-up studies evaluating its efficacy and safety for the treatment of rectus abdominis endometriosis (RAE) (3-9). The present study included the largest number of women with RAE who received HIFU treatment at a gynecological center and involved the longest follow-up period of any study of its type published to date. In addition, we describe 7 years of experience and prognostic evaluation of RAE ablated with HIFU.
The prevalence of AWE is low and is generally estimated to be between 0.03% and 1% (3). RAE is an elusive disease that mainly manifests as periodic pain associated with the menstrual cycle as the primary symptom; however, this mass is often not palpable to the touch, as it is located in deeper tissue (10,11). When the ectopic endometrium grows, periodic bleeding and fibrosis change under the action of menstrual cycle hormones, eventually forming a relatively large palpable painful mass (10,11). However, due to its high recurrence rate (0–29%), abdominal wall defects, and the policy for the cesarean section rate for multiple births (9-11), HIFU is a safer noninvasive modality that is urgently needed in the clinic.
The present study included 56 patients with RAE ablated with HIFU. The demographic characteristics and related factors affecting prognosis were retrospectively analyzed to provide an important reference for the study of RAE and further clarify the potential therapeutic algorithms that can be used in clinical practice. We present the following article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-695/rc).
Methods
Fifty-six patients with AWE treated at the Beijing Obstetrics and Gynecology Hospital from March 2014 to August 2021 were included. We retrospectively analyzed the patients’ HIFU treatment data. All patients signed surgical and angiographic notices before surgery. The inclusion criteria, preoperative preparation, and intraoperative monitoring were detailed in previous studies (3,5).
We used a gynecologic professional HIFU therapeutic system (JC200D; Chongqing Haifu Medical Corporation Ltd., Chongqing, China). An ultrasonic guidance machine (Mylab79, Esaote, Italy) was located in the center of the sink. The treatment probe had the following characteristics: diameter, 20 cm; frequency, 0.8 MHz; emission energy, 24–260 W; and focal region size, 1.5 mm × 1.5 mm × 8 mm. Patients were placed in the prone position, and their anterior abdominal walls were immersed in cold degassed water (temperature <10 ℃). The extent of ablation of the lesion was 1 cm from the lesion and its surrounding area, where the focus was controlled as close to the abdominal cavity as possible, with a safe distance from the focus to the skin being maintained at 8–10 mm.
No other foreign material was present in the acoustic pathway. The rhythm of adjustment was based on the patient’s skin reaction to the thermal radiation and skin changes (5). The results were evaluated in the non-perfused range of contrast-enhanced ultrasound (CEUS). The CEUS and posttreatment observation and management procedures were similar to those described in previous studies (1,4,5). The patients were followed up for 1–84 months after HIFU, and the adverse reactions or complications were recorded according to the standards of the International Society of Radiology (ISR) (4,5).
All data are expressed as the mean ± standard deviation (SD). Statistical analysis was performed using the Student t-test and SPSS software version 21.0 (IBM Corp., Armonk, NY, USA) for self-contrast. P<0.05 was considered statistically significant. The study protocol was approved by the ethics board of the Beijing Obstetrics and Gynecology Hospital and was performed in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was obtained from all of the patients.
Results
A total of 56 patients with 65 RAE lesions with the following characteristics were selected: average age, 36.5±9.19 years; body mass index (BMI), 22.88±5.42 kg/m2; cesarean delivery time, 72±33.49 months; interval from cesarean delivery to RAE diagnosis, 60 ±32.53 months; and visual analog scale (VAS) score, 8.0±0.00 (Table 1). Of these 56 patients, 94.1% (52/56) underwent transverse cesarean sections, while 5.9% (4/56) underwent vertical cesarean sections. The texture of the lesion was hard in 100% of patients (56/56), and the degree of activity was high in 15.9% (9/56) and low in 82.5% (47/56). Approximately 68.4% of patients (39/56) exhibited adhesions to the rectus or had lesions located in the rectus, 26.3% (15/56) had lesions located in the subcutaneous fat layer, and 3.5% (2/56) had adhesions to the fascia. Pain on touch was experienced in 92.9% (61/65) of patients, with a pain score of 3.00±1.4, and the remaining patients experienced no pain before treatment. Skin protrusion was seen in 46.6% of patients (26/56).
Table 1
| Variables | Values |
|---|---|
| No. of patients | 56 |
| No. of lesions | 65 |
| Age (years), mean ± SD [range] | 36.5±9.19 [27–45] |
| BMI (kg/m2), mean ± SD [range] | 22.88±5.42 [17.19–33.02] |
| <18.5 (underweight), n (%) | 2 (3.6) |
| 18.5–24.9 (normal weight), n (%) | 44 (78.6) |
| 25–29.9 (overweight), n (%) | 7 (12.5) |
| >30 (obese), n (%) | 3 (5.4) |
| Time of cesarean delivery (months), mean ± SD [range] | 72±33.49 [24–144] |
| Time interval from the last cesarean delivery (months), mean ± SD [range] | 60±32.53 [1–84] |
| Number of cesarean incisions, n (%) | |
| Two | 8 (12.3) |
| One | 48 (85.7) |
| Desire to give birth again, n (%) | |
| Yes | 20 (35.7) |
| No | 31 (55.4) |
| Unsure | 5 (8.9) |
| VAS score, mean ± SD | 8.0±0.00 |
| Type of cesarean section, n (%) | |
| Vertical | 4 (7.1) |
| Transverse | 53 (92.9) |
| Subjective signs, n (%) | |
| Texture of the lesion | |
| Hardness | 65 (100.0) |
| Pain on palpation, n (%) | |
| Yes | 61 (93.9) |
| No | 4 (6.1) |
| Pain score, mean ± SD; median | 3±1.4; 4.0 |
| Protrusion of the skin, n (%) | |
| Yes | 6 (9.2) |
| No | 59 (90.8) |
| Lesion position, n (%) | |
| To the left of the incision | 22 (33.9) |
| At the middle of the incision | 12 (18.5) |
| To the right of the incision | 31 (47.6) |
| Degree of activity, n (%) | |
| High | 0 (0.0) |
| Low | 65 (100.0) |
| Adhesions to the surrounding tissue, n (%) | |
| Rectus abdominis | 65 (100.0) |
| Subcutaneous fat lining | 5 (23.08) |
| Fascial | 1 (1.54) |
| Number of lesions, n (%) | |
| Single lesion | 57 (87.7) |
| Two lesions | 7 (10.8) |
| Three lesions | 1 (1.5) |
BMI, body mass index; SD, standard deviation; VAS, visual analog scale.
As shown in Table 2, before treatment, the nodule volume was 20.2±23.0 cm3, and after treatment for 88.5±34.65 min of total treatment time and 676.5±102.53 s of total sonication, the nonperfused volume was 20.01±22.75 cm3 while the non-perfused rate was 1.33%±0.48%. The sonication time for 1 cm3 was 82.95±17.89 s, and the total sonication volume was 10.65±5.94 cm3. The total energy was 116,575±5,975.05 J, with an energy efficiency factor (EEF) of 10,052.43±702.65 J/cm3, and the sonication intensity was 511.45±269.75 s/h.
Table 2
| Variables | Mean ± SD |
|---|---|
| Lesion volume, cm3 | 20.2±23.0 |
| Non-perfused volume, cm3 | 20.0±24.1 |
| Rate of non-perfused volume, % | 1.33±0.48 |
| Average power | 175±35.36 |
| Total treatment time, min | 88.5±34.65 |
| Total sonication time, s | 676.5±102.53 |
| Total sonication volume, cm3 | 10.65±5.94 |
| Sonication time for 1 cm3, s/cm3 | 82.95±17.89 |
| Sonication intensity, s/h | 511.45±269.75 |
| Total energy, J | 116,575±5,975.05 |
| EEF, J/cm3 | 10,052.43±702.65 |
| Intraprocedural pain | 7.50±3.53 |
| Postprocedural pain | 5.0±2.83 |
HIFU, high-intensity focused ultrasound; EEF, energy efficiency factor.
As shown in Table 3, 12 patients ISR class A skin thermalgia, and 56 patients with pain in the treatment area had ISR class B skin thermalgia, except for 1 who had a first-degree skin burn where the skin red range was about 1 cm and 2 hematuria cases in whom washing water sample urine appeared in the urethral catheter during treatment, and treatment did not start until the cold saline bladder perfusion was administered to clear the urine.
Table 3
| ISR class | Complications | No. |
|---|---|---|
| A | Skin thermalgia | 12 |
| B | Pain in the treatment area | 65 |
| Skin blistering | 1 | |
| Hematuria | 2 |
HIFU, high-intensity focused ultrasound; ISR, International Society of Radiology.
At 84 months of follow-up, none of the patients experienced periodic pain. Their VAS scores decreased significantly from 1.00±1.41 at 1 month and 0.00±0.00 at 6 months to 0.00±0.00 at 12 months, 0.00±0.00 at 24 months, 0.00±0.00 at 36 months, 0.00±0.00 at 48 months, 0.00±0.00 at 68 months, 1.00±1.41 at 72 months, and 0.00±0.00 at 84 months (Table 4). In addition, the rate of decrease in volume increased from 34.87%±36.04% at 1 month to 81.89%±15.69% at 6 months, 96.16%±5.44% at 12 months, 85.25%±20.86% at 24 months, 85.4%±20.6% at 36 months, 100.00%±0.00% at 48 months, 25.34%±18.83% at 60 months, 81.89%±15.69% at 72 months, and 0.00%±0.00% at 84 months.
Table 4
| Variables | 1 month (n=56) | 6 months (n=50) | 12 months (n=50) | 24 months (n=45) | 36 months (n=40) | 48 months (n=28) | 60 months (n=13) | 72 months (n=11) | 84 months (n=7) |
|---|---|---|---|---|---|---|---|---|---|
| VAS | 1.00±1.41 | 0.00±0.00** | 0.00±0.00** | 0.00±0.00** | 0.00±0.00** | 0.00±0.00** | 0.00±0.00** | 1.00±1.41** | 0.00±0.00** |
| Rate of volume decrease (%) | 34.87±36.04 | 81.89±15.69** | 96.16±5.44** | 85.25±20.86** | 85.4±20.6** | 100.00±0.00** | 25.34±18.83 | 81.89±15.69** | 0.00±0.00** |
**, P<0.01. HIFU, high-intensity focused ultrasound; VAS, visual analog scale.
Discussion
RAE is a rare disease that involves extra-pelvic endometriosis where the endometrium and glands are present in the rectus abdominis following gynecological and/or obstetrics surgery. Its clinical symptoms are elusive and diagnosis is difficult, and there is a long period between occurrence and diagnosis (10,11). In our studies, pain to the touch exhibits a median VAS of 4.0. Only 9.23% of patients had lesions located in the rectus abdominis that protruded from the skin surface, which were hard to the touch during the palpation checkup. Periodic pain was the main complaint of these patients, which would become worse especially when coughing or getting up, and particularly during menstruation. Only 8.93% (n=5) of patients felt nothing, while 91.07% (n=51) of patients had a median VAS of 6.0, and the median time to disease onset was 20 months. The symptom of pain was often confused with those experienced during recovery from a cesarean section incision.
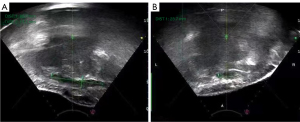
All 2-dimensional (2D) ultrasound images showed hypoechoic lesions with blurred borders, which showed visible blood flow or no obvious blood flow signal around or inside the lesion on color blood flow imaging (12-14). CEUS displayed blood perfusion in all the (15). All endometriotic lesions in the rectus abdominis were shown as solid in 2D ultrasonic imaging (Figure 1). For suspected abdominal organ involvement, MRI is recommended to rule out abdominal organ adhesions. AWE involving muscle and/or fascia requires the en bloc resection of myofascial elements. In these cases, it may be necessary to implant a mesh to repair the defect. Buscemi et al. reported on 46 patients with a painful mass next to the scar, with a mean size of 26.8±13.8 mm on US. Among these cases, mesh implantation was required to repair abdominal wall defects in 7 patients (15.2%), while local resection with direct reconstruction of the muscle fascia was performed in 39 patients (84.8%) (10).
In our series, 56 patients (100%) with 65 lesions all had lesions that were strongly fixed to the myofascial elements of the abdominal wall. During traditional surgery, a wide excision should be performed to reduce the postoperative recurrence, and an en bloc resection requires the implantation of a mesh to repair the defect. Moreover, approximately 35.7% (20/56) of patients wanted to become pregnant again in the future, 55.36% (31/56) did not want a second child, and 5 (8.9%) were unsure. The possibility of a further pregnancy must be taken into account in selecting the type of intervention. The presence of a mesh may affect subsequent cesarean procedure selections.
Our results showed that a total of 56 patients with RAE had a history of cesarean section during the reproductive stage (mean age 34 years); 85.7% (48/56) patients had at least 1 cesarean section, and 14.3 % (8/56) patients had 2 cesarean sections. About 78.57% of the patients were of normal weight, and 3.57% of the patients were underweight. The safe distance from the thermal focus point to the skin surface is at least 8–10 mm. All of the lesions were very close to the peritoneum. During the procedure, the patients were in the prone position and reactively held their breath with contractions due to burning pain. Otherwise, the thermal focus should be set as close as possible next to the back field.
A HIFU device was used to evaluate the safety of the ablation path before treatment. All 65 lesions were confirmed, which were 100% solid, as confirmed by ultrasound. In addition, 87.69% (57/65) of patients had a single lesion, 10.77% (7/65) had 2 lesions, and 1.54% (1/65) had 3 lesions. All (65/65) lesions exhibited low activity. In terms of adhesions to surrounding tissues, all (65/65) of the lesions had adhesions at the rectus, 23.08% (5/65) had adhesions to the subcutaneous fat tissue, and 1.54% (1/65) had fascial adhesions. The median lesion diameter was 26 mm (range, 8–67 mm). The median lesion diameter thickness was 20 mm (range, 11–37 mm). RAE with muscle involvement that is not treated will not only continue to develop but can also grow toward the abdominal cavity and wrap and adhere to the abdominal organs. Ablation is feasible with HIFU regardless of the amount of pain or the size as long as it is visible and safe to perform in the ultrasonic path under imaging guidance (3,5,6). Ultrasound (12-14) plays an important role in confirming the presence of lesions regardless of how small the lesion is. It is also important in determining the location, size, texture, margin, and number of RAE lesions, as well as distinguishing between cystic and solid tumors for diagnosis and treatment guidance. There is an imaging difference between high- and low-frequency RAEs. Low-frequency RAEs exhibit strong penetrating power, especially in patients with a thick abdominal wall. Among our patients, 7 (7/56) were overweight (BMI 25–29.9 kg/m2) and 3 were obese (BMI >30 kg/m2). High-frequency RAEs have a strong resolution, which displays a difference between the lesion and surrounding tissue. For superficial lesions, different ultrasound techniques must be combined to make a final comprehensive diagnosis. Considering that the lesion may invade the abdominal organs, we instructed the patients to perform deep-breathing exercises and observed the movement of organs behind the abdominal lesions. Ultrasound has a pivotal effect on the diagnosis of abdominal wall rectus abdominis. The adhesion of the lesion to the abdominal organs can be additionally considered, but MRI is either rarely used or not consequential in this regard.
HIFU conformally ablates the target lesion without affecting the surrounding normal tissues regardless of the size, location, or number of lesions (1-9,15). In our retrospective analysis of 56 patients with RAE and a follow-up time of 1–84 months, 65 lesions were induced to HIFU ablation. The median treatment time was 46 min (17–113 min), the median ablation time was 393 s (71–1,200 s), the median ablation volume was 12.6 cm3 (2.31–281.25 cm3), and the median EEF median was 10,952.38 J/cm3 (6,082.5–182,988.58 J/cm3). The focus volume was 5.0 cm3 (1.35–12.23 cm3), and the deposition energy of unit volume ablation was low. The median posttreatment VAS was 2 (range, 0–7), which disappeared about 2 hours after treatment without any interventions. At 1 month postoperatively, the periodic pain of all patients subsided, the median palpation sensitivity of the VAS score decreased from 3.5 to 1, the lesion volume reduction rate was 34.87%±36.04%, and the adjacent tissue was affected by thermal radiation swelling. During the 1- to 6-month follow-up period, the palpable sensitivity of the VAS score was improved from 1 to 0, and the reduction rate of lesion volume increased from 81.89%±15.69% to 96.16%±5.44%. Over time, the rate of lesion volume reduction gradually accelerated until the lesion completely disappeared at 84 months. Moreover, in the follow-ups at 24, 36, 48, 60, 72, and 84 months, there was no obvious pain and the mass gradually had reduced and finally subsided.
Before the procedure, there were 4 patients with lesions >5 cm in length for the shrinkage of 2- or 3-dose injections of a gonadotropin-releasing hormone (GnRH) analog. Since RAE in is located deep in the rectus abdominis, the symptoms are obscure, and the size is often large when detected. GnRH can not only decrease the growth of the lesion, but can also reduce the vascularity in the lesion via apoptosis. These data are being collected for further analysis. Moreover, this technique has no risk of inducing iatrogenic dissemination of the ectopic endometrium, does not require a patch to prevent abdominal wall defects, and does not affect childbearing or the choice of delivery method, especially for patients who desire pregnancy in the future. For larger lesions and longer ablation time, postoperative pigmentation may occur; however, this will lessen or fade with recovery. In general, patients can move freely and return to normal life and work but may experience skin burns and pain in the treatment area during treatment and swelling and pain that lasts about 2 hours after treatment.
The advantages of HIFU ablation compared to a surgical incision for RAE lesions include a significantly shorter hospital or outpatient stay, no bleeding or dissemination, and a noninvasive nature. Its biggest disadvantage is the presence of the lesion ablated in the abdominal wall without non-pathological results; however, this may also be another advantage, as it can allow for the abdominal wall to remain intact without a patch. Moreover, given that it is a noninvasive procedure, it can be repeated if the lesion recurs. Although regular postoperative follow-up has clearly demonstrated the risk of malignancy, longer-term observations are needed to confirm the results of hyperthermia. A previous systematic review on the surgical resection of AWE lesions reported recurrence rates ranging from 0% to 29% (9). The postoperative recurrence rate of AWE lesions in China ranges from 1.5% (2/29) to 9.9% (10/101) (16). Recently, a study comparing HIFU treatment and surgery showed that the recurrence rate of AWE in the HIFU group was 4.4% (1/23) after 1 year, and these patients needed wider surgical resection (7).
To date, no studies have reported the recurrence rate of RAE. The present study showed that the recurrence rate of HIFU in the treatment of patients with RAE was 1.8% (1/56). One patient who had a cesarean section more than 9 years prior had periodic pain in the right lower abdomen for more than 7 years, which had worsened over the previous 4–5 years, with a VAS score of 8. No cause was identified for the periodic pain in the right lower abdomen, menstrual pain, or postmenstrual pain, which lasted for about 10 days and was tolerable. The patient self-reported and attended several hospitals, and adhesions after cesarean section without treatment was considered. Her pain worsened over the previous 4–5 years and could last for 15–20 days during menstruation and postmenstruation; she took analgesics orally by herself (the specific medications and dosages are unknown).
The patient then went to a traditional Chinese hospital again, and she was considered to have pelvic inflammatory disease; she was given oral Chinese medicine treatment (the specific medications and dosages are unknown), which proved ineffective. Subsequently, she was diagnosed with the same disease and given similar Chinese patent medicines for symptomatic treatment in various hospitals, which also proved ineffective. After hospitalization, she was given traditional Chinese medicine and physical therapy for pelvic inflammatory disease. On the second day of hospitalization, abdominal ultrasound showed that there was a hypoechoic area, about 3 cm in diameter, in the muscle layer of the right incision in the lower abdomen, which was considered AWE. The doctor suggested that she should go to a higher-level hospital. On November 25, 2015, she received HIFU treatment in our hospital. Given this patient’s treatment process, it is likely that a diagnosis was not made for such a prolonged period because the symptoms remained hidden.
Since the patient’s intraoperative pain was obvious, it was difficult for her to tolerate the procedure, and we constantly adjusted the treatment rhythm and strategy to complete the planned process as soon as possible. The total treatment time was 196 min, the ablation time was 448 seconds, the total energy was 87,950 J, the ablation area was 8.7 cm3, the EEF was 51.5 s/cm3, and the ablation rate was 96.56%. During the treatment, the patient’s VAS was 10. At 1 month postoperatively, the lesion had shrunk to 37 mm × 27 mm × 17 mm, and the VAS had decreased to 6. However, the pain relief was not obvious. In September 2016, the lesion was removed through surgery with a patch via telephone follow-up. Despite intraoperative sedation and analgesia, the median intraoperative pain score 7, which was higher than the patient’s preoperative pain (VAS =6).
In this study, 53.6% (30/56) of patients received treatment for pain with a score >7 points, among whom 9 had a score of 10 points, 6 had a score >9 points, 5 had a score >8 points, and 10 had a score >7 points. During the treatment, we adopted several measures to alleviate the patients’ thermal pain without medicine. We also played music to relieve the suffering of the patient. Comparing different styles of music, we adopted a cyclical playback of Buddhist music to stabilize the patient’s mood and allow the treatment to proceed. Although all patients successfully completed the treatment, the doctors needed to constantly adjust the treatment algorithm and console the patients during the treatment. If there is no pain associated with the treatment or the pain can be improved, this procedure can be performed more smoothly and patients may be more willing to cooperate with the treatment. Additionally, if the patient feels better, they may be willing to choose and accept this procedure. Moreover, the risks of the procedure can be minimized during the treatment.
In this retrospective study, until 7 years of follow-up, the cycle of pain during the menstrual phase was associated with reduced lesions located in the rectus abdominis. The following factors should be considered: (I) nodules infiltrating the muscles or fascia near the peritoneum; (II) pain during the HIFU procedure; and (III) low heat preventing damage to the intestines, bladder, and the skin. In addition, the ablated lesions are often large and extensively invade the rectus muscle, especially the surrounding normal tissue. The incidence of endometriosis in the rectus abdominis remains very low, with the scarce literature on this subject mainly consisting of case reports.
Therefore, the information collected on the recurrence rate of RAE may involve bias and errors. Our study is retrospective; therefore, due to bias, errors, or lack of consistent follow-up, we cannot draw definitive conclusions about the incidence or recurrence rate of RAE after HIFU ablation.
Conclusions
The symptoms of RAE are insidious, and RAE often remains difficult to diagnose clinically for an extended period of time. Typically, imaging is needed to confirm the diagnosis, and medicine may help shrink the lesions. Regardless of the size, location, or number of lesions, HIFU treatment can destroy the lesion, ensure the integrity of the rectus abdominis, and ameliorate the cyclical pain and palpable sensitivity of patients with RAE. The efficacy and safety of HIFU are superior to those of other noninvasive treatments (3-9), and the lesions disappear following ablation. However, this study had some limitations related to its small sample size and retrospective design. Further research is needed to refine the surgical process, improve the intraoperative patient experience, and formulate comprehensive treatment strategies according to the characteristics of the masses.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-695/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-695/coif). The authors have no conflicts of interest to declare.
Ethics Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol was approved by the ethics board of Beijing Obstetrics and Gynecology Hospital and was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was obtained from all of the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen J, Chen W, Zhang L, Li K, Peng S, He M, Hu L. Safety of ultrasound-guided ultrasound ablation for uterine fibroids and adenomyosis: A review of 9988 cases. Ultrason Sonochem 2015;27:671-6. [Crossref] [PubMed]
- Xiao-Ying Z, Ying-Shu G, Jiu-Mei C, Jin-Juan W, Hong Y, Chun-Yi Z, Hua D. Effect of pre-treatment with gonadotropin-releasing hormone analogue GnRH-α on high-intensity focussed ultrasound ablation for diffuse adenomyosis: a preliminary study. Int J Hyperthermia 2018;34:1289-97. [Crossref] [PubMed]
- Xiao-Ying Z, Hua D, Jin-Juan W, Ying-Shu G, Jiu-Mei C, Hong Y, Chun-Yi Z. Clinical analysis of high-intensity focussed ultrasound ablation for abdominal wall endometriosis: a 4-year experience at a specialty gynecological institution. Int J Hyperthermia 2019;36:87-94. [Crossref] [PubMed]
- Marinova M, Huxold HC, Henseler J, Mücke M, Conrad R, Rolke R, Ahmadzadehfar H, Rauch M, Fimmers R, Luechters G, Cuhls H, Radbruch L, Schild HH, Strunk H. Clinical Effectiveness and Potential Survival Benefit of US-Guided High-Intensity Focused Ultrasound Therapy in Patients with Advanced-Stage Pancreatic Cancer. Ultraschall Med 2019;40:625-37. [Crossref] [PubMed]
- Zhang X, Duan H. One-time high-intensity focused ultrasound ablation of abdominal wall endometriosis with concurrent uterine fibroids or adenomyosis: two cases and literature review. Quant Imaging Med Surg 2020;10:511-7. [Crossref] [PubMed]
- Zhang X, Duan H. Effect of high-intensity focused ultrasound ablation on endometriosis of the abdominal wall. Int J Clin Exp Pathol 2018;11:2118-24. [PubMed]
- Zhu X, Chen L, Deng X, Xiao S, Ye M, Xue M. A comparison between high-intensity focused ultrasound and surgical treatment for the management of abdominal wall endometriosis. BJOG 2017;124:53-8. [Crossref] [PubMed]
- Luo S, Zhang C, Huang JP, Huang GH, He J. Ultrasound-guided high-intensity focused ultrasound treatment for abdominal wall endometriosis: a retrospective study. BJOG 2017;124:59-63. [Crossref] [PubMed]
- Stehouwer BL, Braat MNG, Veersema S. Magnetic Resonance Imaging-Guided High-Intensity Focused Ultrasound is a Noninvasive Treatment Modality for Patients with Abdominal Wall Endometriosis. J Minim Invasive Gynecol 2018;25:1300-4. [Crossref] [PubMed]
- Buscemi S, Maiorana A, Fazzotta S, Incandela D, Palumbo VD, Damiano G, Maffongelli A, Messina M, Bisso C, Anzelmo G, Curione F, Cantavenera V, Bellomo E, Raia VE, Scimeca R, Geraci G, Cudia BM, Lo Monte AI. Scar endometriosis: not a rare cause for a painful scar. Clin Ter 2021;172:129-33. [PubMed]
- Ozkan OF, Cikman O, Kiraz HA, Roach EC, Karacaer MA, Karaayvaz M. Endometrioma localized in the rectus abdominis muscle: a case report and review of literature. Arq Bras Cir Dig 2014;27:304-6. [Crossref] [PubMed]
- Cocco G, Delli Pizzi A, Scioscia M, Ricci V, Boccatonda A, Candeloro M, Tana M, Balconi G, Romano M, Schiavone C. Ultrasound Imaging of Abdominal Wall Endometriosis: A Pictorial Review. Diagnostics (Basel) 2021;11:609. [Crossref] [PubMed]
- Allen SE, Rindos NB, Mansuria S. Abdominal wall endometriosis: an update in diagnosis, perioperative considerations and management. Curr Opin Obstet Gynecol 2021;33:288-95. [Crossref] [PubMed]
- Edwards K, Tsai SH, Kothari A. Clinical and imaging features of abdominal wall endometriomas. Australas J Ultrasound Med 2018;21:24-8. [Crossref] [PubMed]
- Wang S, Li BH, Wang JJ, Guo YS, Cheng JM, Ye H, Zang CY, Zhang Y, Duan H, Zhang XY. The safety of echo contrast-enhanced ultrasound in high-intensity focused ultrasound ablation for abdominal wall endometriosis: a retrospective study. Quant Imaging Med Surg 2021;11:1751-62. [Crossref] [PubMed]
- Ding Y, Zhu J. A retrospective review of abdominal wall endometriosis in Shanghai, China. Int J Gynaecol Obstet 2013;121:41-4. [Crossref] [PubMed]


