
Idiopathic pulmonary fibrosis disease progression: a dynamic quantitative chest computed tomography follow-up analysis
Introduction
Idiopathic pulmonary fibrosis (IPF) is a rare and irreversible interstitial lung disease (ILD) with a median survival time of only 2 to 5 years after diagnosis (1). The evaluation of disease progression in patients with IPF is crucial, as it influences treatment decisions when assessing response to therapy. Currently, the accepted gold standard for defining clinical deterioration in patients with IPF is a decline in forced vital capacity (FVC) (2,3). By contrast, the significance of marginal change (5–10%) is unclear, and sensitivity is low because of its variability (4). Moreover, pulmonary function tests (PFTs) only reflect overall lung function and cannot be performed with patients with severe IPF. Several prior studies have demonstrated that semiquantitative visual assessment of fibrosis extent using high-resolution computed tomography (HRCT) predicts prognosis of IPF (5-7). However, this method is subjective and susceptible to interobserver variability (8). Recently, computer-assisted quantitative CT (QCT) has attracted the attention of researchers, because it has been shown to be more accurate than visual scoring (6). Multiple studies have shown that the extent of lesions on CT correlates with prognosis in IPF (6,9), whereby the term ‘lesions’ encompasses the 4 typical features of IPF seen on CT (honeycombing, reticular pattern, ground glass opacity, and traction bronchiectasis). However, most of these studies focused on lesion extent at baseline. Additionally, pulmonary vessel volume based on QCT has been reported to be associated with disease severity and prognosis in IPF (10). The current study aims to explore changes in lesion extent ratios at different sites in the lungs as well as changes in pulmonary vessel-related parameters on CT between the 2 patient groups based on changes in their PFTs. The following article is presented in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-843/rc).
Methods
Study cohort and design
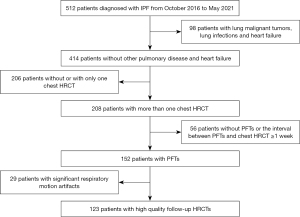
All patients were included retrospectively if they were diagnosed with IPF at the Department of Pulmonary and Critical Care Medicine, China-Japan Friendship Hospital, by a multidisciplinary team between October 2016 and May 2021, and if they were followed-up up until December 2021. Diagnoses were based on the 2011 American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and Latin American Thoracic Association (ATS/ERS/JRS/ALAT) criteria. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Board of the China-Japan Friendship Hospital (No. 2017-25), and individual consent for this retrospective analysis was waived. The inclusion criteria were as follows: (I) patients who underwent at least 2 thin-section (high-resolution) CT chest scans; and (II) diagnosis of PFTs occurred within 1 week of completing both HRCTs. The exclusion criteria were as follows: (I) comorbid pulmonary diseases such as malignant tumors and lung infections; (II) heart failure; and (III) significant respiratory motion artifacts. The flowchart was shown in Figure 1.
CT protocol
All cases were scanned from the lung apex to the lung base in a supine position with full inspiration using a multislice spiral CT scanner (Lightspeed VCT/64, GE Healthcare, Chicago, IL, USA; Toshiba Aquilion ONE TSX-301C/320, Toshiba, Tokyo, Japan; Philips iCT/256, Philips, Amsterdam, The Netherlands; and Siemens FLASH Dual Source CT, Siemens, Erlangen, Germany). The acquisition and reconstruction parameters of HRCT scans were as follows: tube voltage of 100–120 kV, tube current of 100–300 mAs, section thickness setting of 0.625 to 1 mm, table speed of 39.37 mm/s, gantry rotation time of 0.8 s, and reconstruction increment of 1 mm.
Lesion segmentation and quantification
A deep learning approach (InferReadTM CT Lung, version R3.12.3; Infervision Medical Technology Co., Ltd., Beijing, China) was used for lung segmentation and calibration and was performed by 2 radiologists with 9 and 15 years of clinical experience. The lungs were trisected vertically and divided in the axial plane into right upper lung, right middle lung, right lower lung, left upper lung, left middle lung, and left lower lung (i.e., not by anatomical lung lobes). The 4 typical imaging features of IPF on chest HRCT (honeycombing, reticular pattern, ground glass opacity, and traction bronchiectasis) were manually segmented together by the same 2 radiologists. The lesion volumes at different sites were automatically measured by computer, and the percentage of lesion extent was calculated according to lung volumes.
Vessel segmentation and quantification
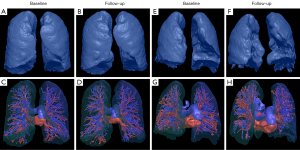
Automatic segmentation of pulmonary vessels on HRCT was performed with an automated integrated segmentation method according to our previously reported method (11). Vessel parameters measured included total pulmonary vessel volume (TPVV), pulmonary vein volume (PVV), pulmonary artery volume (PAV), and the number of vessels (Figure 2). The following steps were performed: (I) recognition of intrapulmonary vessels by means of a computational differential geometry scheme; (II) skeletonization of intrapulmonary vessels to aid in the tracing of adjacent vessel branches; (III) the intrapulmonary vascular skeleton was traced to distinguish arteries and veins originating from extrapulmonary arteries and veins; (IV) the automatic segmentation results were checked and manually corrected by the radiologist.
Pulmonary function testing
All patients underwent PFTs and HRCT during the same week. Parameters for the study included percentage of predicted vital capacity (VC%), percentage of predicted forced vital capacity (FVC%), percentage of predicted forced expiratory volume in 1 second (FEV1%), percentage of predicted total lung capacity (TLC%), and percentage of predicted diffusing lung capacity for carbon monoxide (DLco%). In this study, patients with a 10% absolute decrease in FVC% or 15% absolute decrease in DLco% were classified as showing disease progression, otherwise they were classified as functional stability according to the ATS/ERS/JRS/ALAT 2011 guideline (1).
Statistical analysis
All data were analyzed with SPSS 26.0 software (IBM Corp., Armonk, NY, USA). Differences between groups in the study population were compared with Student’s t-test for quantitative data or the chi-squared test for categorical variables. Correlations between the indicators including pulmonary vessel-related parameters, pulmonary lesion extent, and PFTs were analyzed using Pearson/Spearman Rho correlation coefficients. P values were 2-tailed, and results with P<0.05 were considered statistically significant.
Results
Demographics
A total of 123 eligible patients with IPF diagnosed at the China-Japan Friendship Hospital between 2016 and 2021 were included in this study. Patients were divided into 2 groups based on PFTs: those with functional stability (n=84) and those with functional worsening (n=39). The median age of the total population was 62 years [interquartile range (IQR), 59 to 68 years] and the majority (87.0%) of patients were male (n=107). The median body mass index (BMI) was 24.6 kg/m2 (IQR, 23.4 to 26.3 kg/m2).
In the total population, the median baseline FVC% was 87.1% (IQR, 74.5% to 97.2%) and 43.1% of patients (n=53) showed a decrease in FVC% (<80% of the predicted value). Meanwhile, the median DLco% was 56.2% (IQR, 46.4% to 68.6%), and 78.0% of patients (n=96) exhibited a decrease in DLco% (<70% of the predicted value). There were no statistically significant differences in baseline PFTs, including VC%, FVC%, TLC%, and DLco%, between the 2 groups (with P values ranging from 0.406 to 0.839) (Table 1). Additionally, the median FVC% and DLco% decreased more significantly in those with functional worsening compared with those with functional stability according to the ATS/ERS/JRS/ALAT 2011 criteria [FVC%, −20.0% (IQR, −25.1% to −11.9%) vs. −2.4% (IQR, −5.3% to 3.1%), respectively, P<0.001; and DLco%, −18.6% (IQR, −22.5% to −13.9%) vs. 0.7% (−4.6% to 6.4%), respectively, P<0.001] (Table 1).
Table 1
| Characteristics | Total patients (n=123) | Patients with functional stability (n=84) | Patients with functional worsening (n=39) | P value |
|---|---|---|---|---|
| Median age (years)* | 62 (59 to 68) | 62 (59 to 68) | 63 (56 to 68) | 0.854 |
| Male, n (%) | 107 (87.0) | 75 (89.3) | 32 (82.1) | 0.267 |
| BMI (kg/m2) | 24.6 (23.4 to 26.3) | 25.4 (23.6 to 28.4) | 24.0 (22.6 to 24.9) | 0.002 |
| Median baseline pulmonary function test results* | ||||
| VC% | 85.4 (74.2 to 93.5) | 85.6 (72.0 to 94.0) | 80.5 (74.9 to 90.8) | 0.802 |
| TLC% | 70.0 (62.2 to 79.5) | 72.3 (59.7 to 79.7) | 66.9 (63.7 to 77.9) | 0.839 |
| FVC% | 87.1 (74.5 to 97.2) | 86.7 (74.1 to 97.7) | 89.8 (74.9 to 93.6) | 0.673 |
| DLco% | 56.2 (46.4 to 68.6) | 56.2 (46.2 to 67.8) | 57.5 (46.4 to 69.3) | 0.406 |
| Median time intervals between baseline and follow-up CT (months)* | 10.0 (4.6 to 24.3) | 8.2 (3.8 to 20.2) | 20.6 (8.3 to 24.8) | 0.004 |
| Median changes of pulmonary function test results* | ||||
| VC% | −4.6 (−8.2 to 2.1) | −2.3 (−5.1 to 2.7) | −13.5 (−25.6 to −12.3) | <0.001 |
| TLC% | −1.2 (−5.2 to 3.8) | 2.0 (−2.1 to 7.6) | −8.8 (−17.9 to −4.3) | <0.001 |
| FVC% | −5.0 (−11.8 to 2.4) | −2.4 (−5.3 to 3.1) | −20.0 (−25.1 to −11.9) | <0.001 |
| DLco% | −4.4 (−13.9 to 2.5) | 0.7 (−4.6 to 6.4) | −18.6 (−22.5 to −13.9) | <0.001 |
*, numbers in parentheses represent the interquartile range. BMI, body mass index; VC%, percentage of predicted vital capacity; TLC%, percentage of predicted total lung capacity; FVC%, percentage of predicted forced vital capacity; DLco%, percentage of predicted diffusing lung capacity for carbon monoxide.
Quantitative analysis of different lesion sites
There were no statistically significant differences in baseline median lung volume and total lesion volume between patients with functional stability and functional worsening (P>0.05).
The median lesion volume ratio in the right lower lung was significantly higher than that in the right middle and right upper lungs at 27.50% (IQR, 15.58% to 46.17%), 6.61% (IQR, 3.80% to 13.36%), and 3.81% (IQR, 0.99% to 14.20%), respectively. The same phenomenon was observed on the left lung (Table 2). Additionally, there were significant negative correlations between the baseline total lesion volume ratio and the ratio of lesions at different sites (including right upper, right middle, right lower, left upper, left middle, and left lower lung) with DLco% (with R ranging from −0.66 to −0.46, P<0.001) (Table S1).
Table 2
| Characteristics | Total patients (n=123) | Patients with functional stability (n=84) | Patients with functional worsening (n=39) | P value |
|---|---|---|---|---|
| Total lung volume (mL) | 3,808.23 (3,414.65 to 4,606.59) | 3,770.14 (3,359.05 to 4,587.80) | 4,148.06 (3,531.61 to 4,993.88) | 0.304 |
| Total lesion volume (mL) | 479.25 (226.98 to 764.31) | 496.05 (255.94 to 757.45) | 455.23 (211.98 to 856.28) | 0.894 |
| Total lesion volume ratio (%) | 12.06 (6.15 to 19.12) | 11.05 (6.56 to 18.94) | 13.79 (5.30 to 21.38) | 0.676 |
| Extent of lesions at different sites | ||||
| Right upper lung lesion volume (mL) | 23.13 (4.57 to 65.38) | 20.76 (4.49 to 83.21) | 26.16 (5.20 to 55.87) | 0.183 |
| Right upper lung lesion volume ratio (%) | 3.81 (0.99 to 14.20) | 3.53 (0.90 to 14.79) | 4.65 (0.99 to 14.20) | 0.243 |
| Right middle lung lesion volume (mL) | 73.72 (39.55 to 152.51) | 87.76 (45.87 to 177.35) | 73.72 (27.57 to 93.67) | 0.107 |
| Right middle lung lesion volume ratio (%) | 6.61 (3.80 to 13.36) | 7.03 (4.32 to 17.24) | 6.45 (2.01 to 10.0) | 0.079 |
| Right lower lung lesion volume (mL) | 116.05 (67.41 to 189.82) | 112.41 (75.85 to 186.23) | 137.65 (65.35 to 230.35) | 0.380 |
| Right lower lung lesion volume ratio (%) | 27.50 (15.58 to 46.17) | 29.41 (13.53 to 49.86) | 27.49 (16.22 to 43.73) | 0.555 |
| Left upper lung lesion volume (mL) | 10.59 (4.12 to 28.36) | 10.93 (4.05 to 34.53) | 7.78 (4.12 to 28.36) | 0.140 |
| Left upper lung lesion volume ratio (%) | 2.40 (0.57 to 4.71) | 2.43 (0.75 to 5.84) | 1.84 (0.51 to 4.71) | 0.146 |
| Left middle lunge lesion volume (mL) | 67.80 (22.98 to 121.69) | 70.91 (31.09 to 118.98) | 55.75 (17.77 to 146.28) | 0.694 |
| Left middle lunge lesion volume ratio (%) | 7.78 (2.98 to 15.90) | 7.96 (3.07 to 16.32) | 5.08 (2.78 to 15.53) | 0.453 |
| Left lower lung lesion volume (mL) | 88.61 (34.39 to 181.51) | 88.22 (27.64 to 159.95) | 153.58 (44.66 to 199.57) | 0.057 |
| Left lower lung lesion volume ratio (%) | 29.79 (9.39 to 46.50) | 29.09 (5.54 to 46.07) | 41.17 (12.54 to 49.43) | 0.217 |
Numbers in parentheses represent the interquartile range.
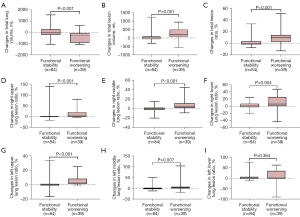
During follow-up, a significantly greater reduction in median total lung volume was observed in patients with functional worsening than in patients with functional stability [−278.01 mL (IQR, −901.64 to −91.54 mL) vs. −40.76 mL (IQR, −212.45 to 286.86 mL), P<0.001] and the median total lesion volume ratio increased significantly [9.38% (IQR, 2.47% to 14.0%) vs. −0.13% (IQR, −2.03% to 3.24%), P<0.001] (Table 3 and Figure 3A-3C). Among different lesion sites, the changes in median lesion volume in the right upper lungs and right middle lungs increased more significantly for patients with functional worsening than those with functional stability. Along the same lines, median changes of lesion ratios in the right upper and right middle lungs were significantly different between the 2 groups [right upper lung, 5.40% (IQR, 0.30% to 21.02%) vs. 0.07% (IQR, −1.50% to 2.0%), P<0.001; right middle lung, 3.84% (IQR, 1.88% to 11.65%) vs. −0.07% (IQR, −2.86% to 2.47%), P<0.001], with similar results observed in the left upper and left middle lungs. However, in the left lower lungs, the differences were not statistically significant (P=0.364), although the extent of lesions increased markedly in the group with progressive disease compared with the stable group (Table 3 and Figure 3D-3I). Moreover, baseline total lesion volume ratio was negatively correlated with DLco% (R=−0.66, P<0.001) (Table S1). In addition, changes in total lesion volume ratio were negatively correlated with changes in DLco% during follow-up (R=−0.57, P<0.001) (Table S2).
Table 3
| Characteristics | Total patients (n=123) | Patients with functional stability (n=84) | Patients with functional worsening (n=39) | P value |
|---|---|---|---|---|
| Total lung volume (mL) | −91.54 (−457.20 to 118.44) | −40.76 (−212.45 to 286.86) | −278.01 (−901.64 to −91.54) | <0.001 |
| Total lesion volume (mL) | 50.78 (−33.17 to 169.36) | 3.67 (−71.70 to 85.33) | 214.73 (68.26 to 501.46) | 0.001 |
| Total lesion volume ratio (%) | 1.83 (−1.35 to 8.28) | −0.13 (−2.03 to 3.24) | 9.38 (2.47 to 14.0) | <0.001 |
| Extent of lesions at different sites | ||||
| Right upper lung lesion volume (mL) | 3.94 (−1.24 to 10.91) | 1.17 (−5.60 to 7.11) | 17.30 (2.14 to 71.43) | 0.004 |
| Right upper lung lesion volume ratio (%) | 0.46 (−0.20 to 2.86) | 0.07 (−1.50 to 2.00) | 5.40 (0.30 to 21.02) | <0.001 |
| Right middle lung lesion volume (mL) | 6.90 (−11.09 to 42.25) | 1.11 (−32.03 to 19.33) | 43.32 (11.22 to 101.57) | <0.001 |
| Right middle lung lesion volume ratio (%) | 0.78(−1.69 to 4.36) | −0.07 (−2.86 to 2.47) | 3.84 (1.88 to 11.65) | <0.001 |
| Right lower lung lesion volume (mL) | 13.99 (−6.41 to 30.18) | 13.94 (−8.64 to 29.48) | 13.99 (4.77 to 54.41) | 0.174 |
| Right lower lung lesion volume ratio (%) | 1.80 (−1.40 to 8.69) | 1.44 (−2.58 to 7.17) | 6.34 (−0.29 to 25.97) | 0.004 |
| Left upper lung lesion volume (mL) | 3.33 (−1.58 to 12.66) | 0 (−6.96 to 8.06) | 12.41 (3.11 to 27.53) | 0.026 |
| Left upper lung lesion volume ratio (%) | 0.69 (−0.32 to 3.65) | 0.06 (−1.23 to 1.10) | 3.44 (1.09 to 8.19) | <0.001 |
| Left middle lunge lesion (mL) | 6.84 (−15.69 to 30.54) | −0.08 (−16.28 to 19.95) | 18.66 (−6.71 to 64.20) | 0.113 |
| Left middle lunge lesion volume ratio (%) | 0.88 (−1.77 to 4.42) | 0.31 (−2.40 to 2.73) | 4.41 (0.36 to 7.95) | 0.007 |
| Left lower lung lesion volume (mL) | 2.22 (−18.28 to 43.60) | −0.14 (−17.39 to 26.93) | 6.62 (−26.68 to 83.29) | 0.858 |
| Left lower lung lesion volume ratio (%) | 1.72 (−1.93 to 13.73) | 1.58 (−2.96 to 7.32) | 4.28 (−1.31 to 35.92) | 0.364 |
Numbers in parentheses represent the interquartile range.
Quantitative analysis of vessel-related parameters
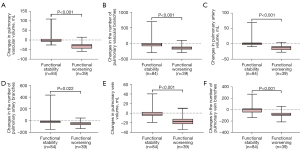
No statistically significant differences were observed between the 2 patient groups for any of the baseline vascular indicators (with P values ranging from 0.066 to 0.964) (Table 4). Nevertheless, baseline pulmonary vessel-related parameters were significantly correlated with DLco% (with R ranging from 0.26 to 0.39, P<0.001) (Table S1). However, TPVV, TPVV ratio, PVV, and PVV ratio, and the number of pulmonary vessel branches were found to decrease significantly in patients with disease progression compared with those with disease stability [TPVV, −28.82 mL (IQR, −43.56 to −22.79 mL) vs. −2.41 mL (IQR, −8.14 to 5.57 mL), respectively, P<0.001; TPVV ratio, −0.67% (IQR, −1.08% to −0.27%) vs. −0.03% (IQR, −0.23% to 0.12%), respectively, P<0.001; and the number of pulmonary vessel branches, −116 (IQR, −178 to −103) vs. −32 (IQR, −79 to 20.5), respectively, P<0.001] (Table 4 and Figure 4). Meanwhile, changes in pulmonary vessel-related parameters demonstrated a statistically positive correlation with DLco% (with R ranging from 0.27 to 0.53, P<0.001) and FVC% (with R ranging from 0.44 to 0.61, P<0.001) (Table S2).
Table 4
| Characteristics | All patients (n=123) | Patients with functional stability (n=84) | Patients with functional worsening (n=39) | P value |
|---|---|---|---|---|
| Median baseline pulmonary vessel-related indexes | ||||
| TPVV (mL) | 91.50 (70.84 to 108.59) | 92.05 (68.64 to 107.35) | 91.50 (73.22 to 110.81) | 0.241 |
| TPVV (%) | 2.34 (1.97 to 2.72) | 2.25 (1.90 to 2.75) | 2.41 (2.21 to 2.67) | 0.340 |
| Number of pulmonary vessel branches | 442 (358 to 489) | 436.50 (355.75 to 479.50) | 454 (362 to 516) | 0.566 |
| PAV (mL) | 48.62 (37.39 to 55.35) | 48.57 (37.07 to 58.15) | 48.67 (37.79 to 53.30) | 0.964 |
| PAV (%) | 1.25 (1.07 to 1.40) | 1.26 (1.04 to 1.43) | 1.19 (1.09 to 1.32) | 0.752 |
| Number of pulmonary artery branches | 244 (205 to 266) | 244 (202 to 269.5) | 244 (210 to 258) | 0.327 |
| PVV (mL) | 42.12 (32.14 to 55.01) | 41.00 (31.82 to 53.25) | 43.23 (32.41 to 59.08) | 0.067 |
| PVV (%) | 1.13 (0.88 to 1.33) | 1.06 (0.84 to 1.33) | 1.18 (1.10 to 1.33) | 0.066 |
| The number of pulmonary vein branches | 193 (147 to 234) | 185.5 (146 to 223) | 196 (152 to 256) | 0.077 |
| Median changes in pulmonary vascular indexes | ||||
| TPVV (mL) | −5.84 (−23.85 to 2.11) | −2.41 (−8.14 to 5.57) | −28.82 (−43.56 to −22.79) | <0.001 |
| TPVV (%) | −0.17 (−0.50 to 0.11) | −0.03 (−0.23 to 0.12) | −0.67(−1.08 to −0.27) | <0.001 |
| Number of pulmonary vessel branches | −52 (−109 to 16) | −32 (−79 to 20.5) | −116 (−178 to −103) | <0.001 |
| PAV (mL) | −2.16 (−9.08 to 2.79) | 0.81 (−3.54 to 3.27) | −11.57 (−20.27 to −8.24) | <0.001 |
| PAV (%) | −0.04 (−0.20 to 0.07) | 0.01 (−0.07 to 0.08) | −0.28 (−0.37 to −0.10) | <0.001 |
| Number of pulmonary artery branches | −24 (−54 to −1) | −14.5 (−34.75 to −1) | −49 (−71 to −27) | 0.022 |
| PVV (mL) | −4.32 (−15.18 to 0.81) | −2.01 (−5.42 to 2.05) | −17.37 (−22.93 to −12.43) | <0.001 |
| PVV (%) | −0.12 (−0.38 to 0.05) | −0.06 (−0.14 to 0.09) | −0.42(−0.73 to −0.17) | <0.001 |
| Number of pulmonary vein branches | −27 (−78 to 13) | −0.5 (−45.5 to 18) | −81 (−102 to −55) | <0.001 |
Numbers in parentheses represent the interquartile range. TPVV, total pulmonary vessel volume; PAV, pulmonary artery volume; PVV, pulmonary vein volume.
Discussion
Visual, semiquantitative parenchymal abnormality scores have been reported in several studies to predict the prognosis of patients with IPF (6,12-14). Nevertheless, the reproducibility of semiquantitative scores is poor and is often limited by interobserver variability (15). The quest for sensitive, reproducible, and objective indicators for accurate assessment of disease progression has therefore received widespread attention (16-18).
HRCT is an essential imaging tool for the diagnosis and follow-up of IPF. PFTs (including FVC and DLco) represent the traditional method of determining disease severity and prognosis (1). This study provides a dynamic quantitative analysis of the extent of lesions and vessel-related parameters on chest CT in patients with IPF. Multiple studies have demonstrated the correlation of quantitative CT-based lesion extent with lung function parameters and, the utility of QCT for determining prognosis in patients with IPF (6,14,19-21). Our research reiterates this, having demonstrated that baseline lesion volume and ratio were negatively correlated with FVC% and DLco% (P<0.001). The same findings were reported for changes in lesion volumes during follow-up. A recent study by Humphries et al. (7) that involved baseline data-driven texture analysis showed that fibrosis scores were significantly associated with annual rates of decline in FVC and DLco. They found that higher fibrosis scores were associated with a poorer prognosis (P<0.001).
Due to a lack of adequate follow-up time, disease progression was assessed in the current study, but not survival outcomes. We found significant differences of lesion extent as well as vascular parameters (pulmonary vessel volume, number of pulmonary vessel branches) over time between the 2 patient groups, whilst there were no statistically significant differences between the 2 groups for these parameters at baseline. This, therefore, suggests that dynamic changes in these imaging findings over time are more reflective of disease severity and prognosis in IPF than findings at baseline.
Several recent studies have demonstrated the potential role of quantitative analysis of vessel-related indicators in the assessment of ILD severity and prognosis (6,10,22,23). In these studies, baseline TPVV, TPVV ratio, and the number of pulmonary vessel branches were not found to be statistically different between groups with functional stability and functional worsening. However, the current study found that changes in these indicators during follow-up were significantly different between these groups. Moreover, baseline pulmonary vessel-related parameters were significantly correlated with DLco% (with R ranging from 0.26 to 0.39, P<0.001). Interestingly, there were stronger correlations between the changes recorded in pulmonary vessel-related parameters and changes in DLco% (with R ranging from 0.27 to 0.53, P<0.001) than with baseline parameters, which further supports the predictive value of dynamic imaging indicators for disease progression and prognosis in IPF.
There are several limitations in this study. Firstly, patients with more severe disease who were unable to complete PFT were excluded from our analysis, which may have led to selection bias. Secondly, many patients failed to complete follow-up because of the impact of the coronavirus disease of 2019 (COVID-19) pandemic, thereby limiting our sample size. Finally, in cases of severe fibrosis, segmentation of pulmonary vessels is susceptible to reticular pattern contamination, which may have mitigated the relationship between pulmonary vessel-related parameters and PFTs.
Conclusions
Our study demonstrates that changes in lung lesion extent and pulmonary vessel-related parameters are significantly different between patients with IPF who display functional stability or functional deterioration according to serial PFTs. Enlargement of pulmonary lesions and reductions in pulmonary vessel volume on CT were the strongest indicators of declining FVC% and DLco%. Therefore, for patients with complete follow-up information and with high-quality chest HRCT, these imaging findings may serve as valuable indicators of disease severity and prognosis in patients with IPF.
Acknowledgments
Funding: This work was supported by the National Key R&D Program of China (Nos. 2021YFC2500700 and 2016YFC0901101 to H Dai).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-843/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-843/coif). HK and RZ are staff of Infervision Medical Technology Co., Ltd. The school/hospital and the company are in a collaborative relationship. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Board of China-Japan Friendship Hospital (No. 2017-25), and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788-824. [Crossref] [PubMed]
- Karimi-Shah BA, Chowdhury BA. Forced vital capacity in idiopathic pulmonary fibrosis--FDA review of pirfenidone and nintedanib. N Engl J Med 2015;372:1189-91. [Crossref] [PubMed]
- Nathan SD, Shlobin OA, Weir N, Ahmad S, Kaldjob JM, Battle E, Sheridan MJ, du Bois RM. Long-term course and prognosis of idiopathic pulmonary fibrosis in the new millennium. Chest 2011;140:221-9. [Crossref] [PubMed]
- Travis WD, Costabel U, Hansell DM, King TE Jr, Lynch DA, Nicholson AG, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733-48. [Crossref] [PubMed]
- Oda K, Ishimoto H, Yatera K, Naito K, Ogoshi T, Yamasaki K, Imanaga T, Tsuda T, Nakao H, Kawanami T, Mukae H. High-resolution CT scoring system-based grading scale predicts the clinical outcomes in patients with idiopathic pulmonary fibrosis. Respir Res 2014;15:10. [Crossref] [PubMed]
- Jacob J, Bartholmai BJ, Rajagopalan S, Kokosi M, Nair A, Karwoski R, Walsh SL, Wells AU, Hansell DM. Mortality prediction in idiopathic pulmonary fibrosis: evaluation of computer-based CT analysis with conventional severity measures. Eur Respir J 2017;49:1601011. [Crossref] [PubMed]
- Humphries SM, Mackintosh JA, Jo HE, Walsh SLF, Silva M, Calandriello L, Chapman S, Ellis S, Glaspole I, Goh N, Grainge C, Hopkins PMA, Keir GJ, Moodley Y, Reynolds PN, Walters EH, Baraghoshi D, Wells AU, Lynch DA, Corte TJ. Quantitative computed tomography predicts outcomes in idiopathic pulmonary fibrosis. Respirology 2022;27:1045-53. [Crossref] [PubMed]
- Watadani T, Sakai F, Johkoh T, Noma S, Akira M, Fujimoto K, Bankier AA, Lee KS, Müller NL, Song JW, Park JS, Lynch DA, Hansell DM, Remy-Jardin M, Franquet T, Sugiyama Y. Interobserver variability in the CT assessment of honeycombing in the lungs. Radiology 2013;266:936-44. [Crossref] [PubMed]
- Romei C, Tavanti LM, Taliani A, De Liperi A, Karwoski R, Celi A, Palla A, Bartholmai BJ, Falaschi F. Automated Computed Tomography analysis in the assessment of Idiopathic Pulmonary Fibrosis severity and progression. Eur J Radiol 2020;124:108852. [Crossref] [PubMed]
- Jacob J, Pienn M, Payer C, Urschler M, Kokosi M, Devaraj A, Wells AU, Olschewski H. Quantitative CT-derived vessel metrics in idiopathic pulmonary fibrosis: A structure-function study. Respirology 2019;24:445-52. [Crossref] [PubMed]
- Sun X, Meng X, Zhang P, Wang L, Ren Y, Xu G, Yang T, Liu M. Quantification of pulmonary vessel volumes on low-dose computed tomography in a healthy male Chinese population: the effects of aging and smoking. Quant Imaging Med Surg 2022;12:406-16. [Crossref] [PubMed]
- Jacob J, Bartholmai BJ, Rajagopalan S, Kokosi M, Egashira R, Brun AL, Nair A, Walsh SLF, Karwoski R, Wells AU. Serial automated quantitative CT analysis in idiopathic pulmonary fibrosis: functional correlations and comparison with changes in visual CT scores. Eur Radiol 2018;28:1318-27. [Crossref] [PubMed]
- Jacob J, Aksman L, Mogulkoc N, Procter AJ, Gholipour B, Cross G, et al. Serial CT analysis in idiopathic pulmonary fibrosis: comparison of visual features that determine patient outcome. Thorax 2020;75:648-54. [Crossref] [PubMed]
- Jacob J, Bartholmai BJ, Rajagopalan S, Kokosi M, Nair A, Karwoski R, Raghunath SM, Walsh SL, Wells AU, Hansell DM. Automated Quantitative Computed Tomography Versus Visual Computed Tomography Scoring in Idiopathic Pulmonary Fibrosis: Validation Against Pulmonary Function. J Thorac Imaging 2016;31:304-11. [Crossref] [PubMed]
- Walsh SL, Calandriello L, Sverzellati N, Wells AU, Hansell DM. Interobserver agreement for the ATS/ERS/JRS/ALAT criteria for a UIP pattern on CT. Thorax 2016;71:45-51. [Crossref] [PubMed]
- Guo L, Yang Y, Liu F, Jiang C, Yang Y, Pu H, Li W, Zhong Z. Clinical Research on Prognostic Evaluation of Subjects With IPF by Peripheral Blood Biomarkers, Quantitative Imaging Characteristics and Pulmonary Function Parameters. Arch Bronconeumol 2020;56:365-72. (Engl Ed). [PubMed]
- Niwamoto T, Handa T, Murase Y, Nakatsuka Y, Tanizawa K, Taguchi Y, et al. Cutaneous T-cell-attracting chemokine as a novel biomarker for predicting prognosis of idiopathic pulmonary fibrosis: a prospective observational study. Respir Res 2021;22:181. [Crossref] [PubMed]
- Kreuter M, Lee JS, Tzouvelekis A, Oldham JM, Molyneaux PL, Weycker D, Atwood M, Kirchgaessler KU, Maher TM. Monocyte Count as a Prognostic Biomarker in Patients with Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med 2021;204:74-81. [Crossref] [PubMed]
- Torrisi SE, Palmucci S, Stefano A, Russo G, Torcitto AG, Falsaperla D, Gioè M, Pavone M, Vancheri A, Sambataro G, Sambataro D, Mauro LA, Grassedonio E, Basile A, Vancheri C. Assessment of survival in patients with idiopathic pulmonary fibrosis using quantitative HRCT indexes. Multidiscip Respir Med 2018;13:43. [Crossref] [PubMed]
- Clukers J, Lanclus M, Mignot B, Van Holsbeke C, Roseman J, Porter S, Gorina E, Kouchakji E, Lipson KE, De Backer W, De Backer J. Quantitative CT analysis using functional imaging is superior in describing disease progression in idiopathic pulmonary fibrosis compared to forced vital capacity. Respir Res 2018;19:213. [Crossref] [PubMed]
- Sun H, Liu M, Kang H, Yang X, Zhang P, Zhang R, Dai H, Wang C. Quantitative analysis of high-resolution computed tomography features of idiopathic pulmonary fibrosis: a structure-function correlation study. Quant Imaging Med Surg 2022;12:3655-65. [Crossref] [PubMed]
- Occhipinti M, Bruni C, Camiciottoli G, Bartolucci M, Bellando-Randone S, Bassetto A, Cuomo G, Giuggioli D, Ciardi G, Fabbrizzi A, Tomassetti S, Lavorini F, Pistolesi M, Colagrande S, Matucci-Cerinic M. Quantitative analysis of pulmonary vasculature in systemic sclerosis at spirometry-gated chest CT. Ann Rheum Dis 2020;79:1210-7. [Crossref] [PubMed]
- Puxeddu E, Cavalli F, Pezzuto G, Teodori E, Rogliani P. Impact of pulmonary vascular volume on mortality in IPF: is it time to reconsider the role of vasculature in disease pathogenesis and progression? Eur Respir J 2017;49:1602345. [Crossref] [PubMed]


