
Multidimensional analysis using low-dose computed tomography to evaluate the severity of Mycoplasma pneumoniae pneumonia in children
Introduction
Mycoplasma pneumoniae pneumonia (MPP) is one of the most common respiratory tract infections in children, accounting for 8–37.5% of all community-acquired pneumonia (CAP) cases (1,2). Although MPP is usually mild and self-limiting, a previous study showed that approximately 12% of children hospitalized for MPP required intensive care (3). However, the best method to accurately predict the clinical progression of MPP remains unclear, and there is great difficulty in making appropriate treatment strategies at the early stage of the disease. Severe cases may require hospitalization for 7 to 14 days or more. Drug cessation and discharge depend on the improvement in the patient’s symptoms, such as afebrile and clinical stability (4). Hence, an accurate and timely assessment of MPP progression is crucial for patients and clinicians.
Mechanisms by which Mycoplasma pneumoniae induces infection include direct effects of the bacteria, indirect immune-mediated effects, and effects mediated through vasculitis or thrombosis secondary to cytokines, chemokines, or immunomodulation (5). Local proliferation of Mycoplasma pneumoniae and the inflammatory response of the lung lead to systemic inflammation (3). Therefore, the degree of local pathological damage to the lung may be closely related to the severity of the Mycoplasma pneumoniae infection. With lung imaging, clinicians can indirectly investigate important manifestations of lung pathology (6). However, to our best knowledge, few studies have multi-dimensionally investigated the association between lung imaging features and MPP severity. This study hypothesizes that certain characteristic features of lung imaging can predict the severity of MPP throughout the body. Chest X-rays fail to demonstrate the details and distribution of lesions, but computed tomography (CT) can clearly show interstitial abnormalities and lobular distribution, such as bronchial wall thickening and reticulonodular or centrilobular nodules, which are common features in patients with MPP (7-9). Thus, chest CT is a favorable method for assessing radiological features of MPP cases, and it can be applied safely in children by adjusting the scanning parameters, such as by using low-dose CT.
Therefore, we aimed to identify stable and reliable low-dose CT features with clinical significance in a cohort study of a large sample of patients with MPP using multidimensional analysis that could precisely predict the severity and progression of MPP and ensure that patients receive timely and reasonable treatment. We present the following article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-508/rc).
Methods
Patients
Between February 2016 and July 2020, 917 children were diagnosed with MPP among 5,112 hospitalized children with CAP in Xinhua hospital. The diagnosis of MPP was based on the following: (I) fever, cough, or auscultatory findings and pulmonary infiltrates visible on chest imaging; and (II) Mycoplasma pneumoniae DNA detected in nasopharyngeal secretions by polymerase chain reaction or ≥4-fold changes in Mycoplasma pneumoniae immunoglobulin (Ig)M and IgG antibody titer between paired acute and convalescent sera, according to the 2018 Infectious Diseases Society of America guidelines of 2018 (10). Patients with CT scans of insufficient quality resulting from motion artifacts, patients without completing CT within 24 hours of admission, patients with mixed infections identified by the detection of respiratory viruses and bacterial cultures, and patients with immunodeficiency and congenital diseases were all excluded. Finally, 752 patients with MPP who underwent low-dose CT within 24 hours of admission were included, and a retrospective cohort study was conducted and analyzed in the study. The CT scan parameters are shown in Table S1. The study flow chart is displayed in Figure 1. This study was approved by the Institutional Ethics Committee of Xinhua Hospital (No. XHEC-D-2021-154), and informed consent was waived because the data in this retrospective analysis were registered anonymously. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
General, clinical, and laboratory characteristics
General information, clinical symptoms, laboratory findings, and clinical outcomes were re-evaluated by infectious disease specialists who reviewed the clinical records of patients enrolled in this study for the following: general characteristics (including sex and age), clinical symptoms [including duration of fever before admission (days)], and the presence of rash, neurological symptoms, encephalitis, and hypoxemia). For each patient, the time of the chest CT was obtained by subtracting the date of onset from the date of the chest CT. Then, the laboratory markers on admission, including lactate dehydrogenase (LDH), interleukin 2-receptor (IL-2R), and C-reactive protein (CRP) level, were reviewed. Finally, clinical outcomes, including duration of fever after admission (days), total duration of fever (days), length of hospital stay (days), refractory pneumonia, intensive care unit treatment, and mortality, were obtained.
Image analyses
Low-dose CT examinations were performed using 64-detector row CT scanner (Brilliance iCT, Philips, Amsterdam, the Netherlands) or a Siemens dual-source CT scanner (Siemens, Munich, Germany) (11). Evaluation of CT features, including consolidation, bronchial wall thickening, nodules, ground-glass attenuation, interstitial reticulation opacities, bilateral pneumonia, atelectasis, lymphadenopathy, and pleural effusion, was guided by the expert consensus according to a Delphi study on image assessment of patients with MPP (Figure 2) (8,9,12-14). Based on the relevant features of consolidation, all patients were further evaluated for the following features: the number of consolidated lung lobes, location of lobar consolidation (upper, middle, or lower right lung lobe; upper or lower left lung lobe), and the occurrence of consolidation without air bronchogram. Two chest radiologists independently assessed each patient’s CT images. Consensus reached by both parties served as the final appraisal report. Disagreements were resolved by consultation with a third chest radiologist with 12 years of experience.
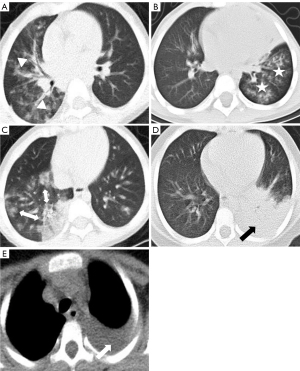
Diagnostic criteria for severe MPP
Each patient’s medical history was re-evaluated by a reviewing expert committee composed of pediatricians, radiologists, and infection specialists. According to the guidelines of the Pediatric Infectious Diseases Society and Infectious Diseases Society of America on the management of CAP in infants and children older than 3 months, diagnosis of severe pneumonia must meet the following: (I) 1 of the major criteria, including invasive mechanical ventilation, fluid refractory shock, urgent need for noninvasive positive pressure ventilation and hypoxemia requiring inspired oxygen; or (II) 2 secondary criteria, including increased respiratory rate, apnea, increased work of breathing, a PaO2 to FiO2 ratio of less than 250, multilobar infiltration, pediatric early warning score >6, altered mental status, hypotension, presence of effusion, comorbidities, and unexplained metabolic acidosis (15). Refractory pneumonia was defined as persistent fever, worsening clinical symptoms, and/or deteriorating radiological findings despite appropriate antibiotic therapy for 7 or more days (16).
Statistical analyses
Categorical variables are expressed as numbers [percentage (%)], and continuous variables are presented as medians [interquartile range (IQR)]. Clinical and laboratory variables in children with MPP were assessed using the chi-squared test for categorical variables and the t-test or Mann-Whitney test for continuous variables. Interobserver agreement in interpreting the CT images was analyzed using Cohen kappa (k) value. The k value was defined as follows: no agreement (<0.00), slight agreement (0.00–0.20), fair agreement (0.21–0.40), moderate agreement (0.41–0.60), substantial agreement (0.61–0.80), and almost perfect agreement (0.81–1.00). Univariate analysis of radiological variables in children with severe MPP (SMPP) was evaluated using the chi-squared test, and the collinearity was evaluated based on the variance inflation factor (VIF). Only those CT features with VIF values of less than 5 and P values of less than 0.001 were selected to assess severe cases in the multivariate logistic regression. The performance of the logistic regression model was evaluated according to accuracy, sensitivity, and specificity. The Kruskal-Wallis H test was used to analyze the association between key CT features and laboratory parameters, as well as medical cost.
Associations between key CT features and disease progression, including fever duration after admission and length of hospital stay, were assessed using Kaplan-Meier estimates and Cox proportional-hazard models to calculate hazard ratios (HRs) with 95% CIs. Missing values in the study mainly included laboratory indicators, accounting for <5% of the total data. Therefore, missing data were not included in the statistical evaluation. A P<0.05 indicated a statistically significant difference. Statistical analysis was performed using R software (https://www.R-project.org; The R Foundation for Statistical Computing, Vienna, Austria).
Results
Clinical and laboratory characteristics of children with SMPP
Of the 752 patients with MPP, 122 (16.2%) had SMPP. All the clinical characteristics of children with MPP stratified by severity of disease are shown in Table 1. Most patients were preschool and school-aged children. Patients with SMPP were more likely to have neurological symptoms, encephalitis, hypoxemia, and refractory pneumonia; they were also more likely to receive treatment in the intensive care unit and have higher LDH, IL-2R, and CRP levels. Furthermore, patients with SMPP also tended to have longer median fever durations after admission, total fever duration, and length of hospital stay than did those without SMPP.
Table 1
| Variables | Non-SMPP (n=630) | SMPP (n=122) | P value |
|---|---|---|---|
| General characteristics | |||
| Male | 298 (47.3) | 56 (45.9) | 0.78 |
| Age (years) | 5.0 (3.0–7.0) | 6.0 (4.0–7.0) | 0.07 |
| Clinical symptoms | |||
| Duration of fever prior admission (days) | 6.0 (5.0–8.0) | 7.0 (5.0–8.0) | 0.82 |
| Rash | 2 (0.3) | 0 (0.0) | 0.53 |
| Neurological symptoms | 0 (0.0) | 9 (7.4) | <0.001 |
| Encephalitis | 0 (0.0) | 2 (1.6) | 0.01 |
| Hypoxemia | 0 (0.0) | 12 (9.8) | <0.001 |
| Laboratory parameters | |||
| LDH (U/L) | 328.5 (282.8–390.2) | 422.0 (332.0–588.0) | <0.001 |
| IL-2R (U/mL) | 961.5 (711.0–1,274.5) | 1,371.5 (972.5–1,985.2) | <0.001 |
| CRP (mg/L) | 11.0 (4.0–22.0) | 18.0 (8.0–39.5) | <0.001 |
| Clinical outcomes | |||
| Duration of fever after admission (days) | 1.0 (0.0–3.0) | 3.0 (2.0–4.0) | <0.001 |
| Total fever duration (days) | 8.0 (6.0–10.0) | 9.0 (7.0–12.0) | <0.001 |
| Length of hospital stay (days) | 6.0 (5.0–8.0) | 9.0 (7.0–12.0) | <0.001 |
| Refractory pneumonia | 164 (26.0) | 61 (50.0) | <0.001 |
| Treatment in the intensive care unit | 0 (0.0) | 7 (5.7) | <0.001 |
| Death | 0 (0.0) | 0 (0.0) | – |
Male, rash, neurological symptoms, encephalitis, hypoxemia, refractory pneumonia, treatment in the intensive care unit, and death are presented as n (%); the rest of the variables are indicated as median (25th−75th percentiles). SMPP, severe Mycoplasma pneumoniae pneumonia; LDH, lactate dehydrogenase; IL-2R, interlukin-2 receptor; CRP, C-reactive protein.
CT features of children with SMPP
Interobserver consistency for CT features varied from substantial agreement to almost perfect agreement (Table 2). In contrast, interobserver consistency for atelectasis and lymphadenopathy was poor. There were 122 cases requiring reassessment by a third chest radiologist. Consolidation was observed in 90.3% of cases (679/752); its incidence was second only to bronchial wall thickening (93.2%; Table 2). The proportions for 1, 2, 3, and 4 lobar consolidations were 65.2% (490/752), 19.3% (145/752), 4.7% (35/752), and 1.2% (9/752), respectively. There were no patients with 5 lobar consolidations. The differences in the number of consolidated lung lobes (VIF values =1.28), consolidations without air bronchogram (VIF values =1.06), atelectasis (VIF values =1.04), and pleural effusion (VIF values =1.00) between MPP and SMPP were statistically significant (P values <0.001) More details are available in Table 2.
Table 2
| CT characteristics | Kappa value | Non-SMPP (n=630) | SMPP (n=122) | P value |
|---|---|---|---|---|
| Consolidation | 1.0 | 557 (88.4) | 122 (100.0) | <0.001 |
| Number of lobes | <0.001 | |||
| 1 | 1.0 | 418 (66.3) | 72 (59.0) | |
| 2 | 1.0 | 113 (17.9) | 32 (26.2) | |
| 3 | 1.0 | 22 (3.5) | 13 (10.7) | |
| 4 | 1.0 | 4 (0.6) | 5 (4.1) | |
| Location | ||||
| Upper lobe, right lung | 1.0 | 109 (17.3) | 34 (27.9) | 0.01 |
| Middle lobe, right lung | 1.0 | 170 (27.0) | 47 (38.5) | 0.01 |
| Lower lobe, right lung | 1.0 | 156 (24.8) | 40 (32.8) | 0.07 |
| Upper lobe, left lung | 1.0 | 144 (22.9) | 30 (24.6) | 0.68 |
| Lower lobe, left lung | 1.0 | 147 (23.3) | 45 (36.9) | 0.002 |
| Without air bronchogram | 0.87 | 54 (9.7) | 35 (28.7) | <0.001 |
| Bilateral pneumonia | 0.94 | 339 (53.8) | 74 (60.7) | 0.16 |
| Pleural effusion | 0.79 | 15 (2.4) | 104 (85.2) | <0.001 |
| Atelectasis | 0.69 | 45 (7.1) | 28 (23.0) | <0.001 |
| Lymphadenopathy | 0.62 | 226 (35.9) | 59 (48.4) | 0.01 |
| Bronchial wall thickening | 0.87 | 588 (93.3) | 113 (92.6) | 0.76 |
| Nodules | 0.83 | 313 (49.7) | 73 (59.8) | 0.04 |
| Ground-glass attenuation | 0.86 | 478 (75.9) | 83 (68.0) | 0.07 |
| Interstitial reticulation opacities | 0.83 | 193 (30.6) | 47 (38.5) | 0.09 |
| The timing of the CT examination during the course of the disease (days) | – | 7.0 (6.0–9.0) | 8.0 (6.0–9.0) | 0.86 |
The timing of the CT examination during the course of the disease is indicated as a median (25th−75th percentiles); the rest of the variables are presented as n (%).SMPP, severe Mycoplasma pneumoniae pneumonia; CT, computed tomography.
The predictive model for SMPP is shown in equation 1. The accuracy, sensitivity, and specificity of the model were 0.951, 0.853, and 0.973, respectively, at a cutoff value of 0.453. Except for pleural effusion [odds ratio (OR) =236.9, 95% CI: 115.8–484.7], the number of consolidated lung lobes was the highest risk for SMPP. Compared with consolidations of 0 and 1 lobe, those with 2, 3, and 4 lobes were associated with a 1.0-, 3.1-, and 7.5-fold increased risk of SMPP, respectively (2 lobes: OR =2.0, 95% CI: 1.3–3.2; 3 lobes: OR =4.1, 95% CI: 2.0–8.5; 4 lobes: OR =8.5, 95% CI: 2.2–32.6; Table 3).
Table 3
| CT characteristics | Non-SMPP (n=630) (%) | SMPP (n=122) (%) | OR (95% CI) | P value | ORa (95% CI) | P valuea |
|---|---|---|---|---|---|---|
| Consolidation | 557 (88.4) | 122 (100.0) | ||||
| Number of lobes | ||||||
| ≤1 | 491 (77.9) | 72 (59.0) | 1.0 | 1.0 | ||
| 2 | 113 (17.9) | 32 (26.2) | 1.9 (1.2–3.1) | 0.01 | 2.0 (1.3–3.2) | 0.003 |
| 3 | 22 (3.5) | 13 (10.7) | 4.0 (1.9–8.4) | <0.001 | 4.1 (2.0–8.5) | <0.001 |
| 4 | 4 (0.6) | 5 (4.1) | 8.5 (2.2–32.5) | 0.002 | 8.5 (2.2–32.6) | 0.002 |
| Without air bronchogram | ||||||
| 0 | 502 (90.3) | 87 (71.3) | 1.0 | 1.0 | ||
| 1 | 54 (9.7) | 35 (28.7) | 3.7 (2.3–6.1) | <0.001 | 3.7 (2.3–6.0) | <0.001 |
| Pleural effusion | ||||||
| 0 | 615 (97.6) | 18 (14.8) | 1.0 | 1.0 | ||
| 1 | 15 (2.4) | 104 (85.2) | 236.9 (115.8–484.7) | <0.001 | 245.2 (117.9–510.1) | <0.001 |
| Atelectasis | ||||||
| 0 | 585 (92.9) | 94 (77.0) | 1.0 | 1.0 | ||
| 1 | 45 (7.1) | 28 (23.0) | 3.9 (2.3–6.5) | <0.001 | 3.9 (2.3–6.6) | <0.001 |
a, adjusted for age and sex. OR and 95% CI are presented to show the association between patients with CT characteristics and SMPP, as compared with patients without such CT characteristics. SMPP, severe Mycoplasma pneumoniae pneumonia; CT, computed tomography; OR, odds ratio; CI, confidence interval.
The formula for the regression model was as follows: logistic regression model = –3.945 + 1.177 × multilobar_consolidation (2 or more lobes) + 0.559 × consolidation_without_air_bronchogram + 5.374 × pleural_effusion + 0.261 × atelectasis (Eq. [1]).
Stability and reliability of key CT features
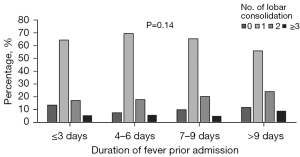
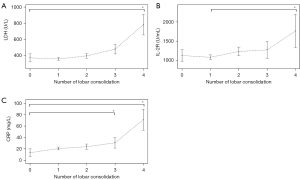
The pleural effusion rates in patients with preadmission fever durations of 3 or fewer, 4–6, 7–9, and more than 9 days were 3.5% (4/115), 14.5% (40/276), 21.1% (50/236), and, 20% (25/125) respectively. The consolidation rates in patients with preadmission fever durations of 3 or fewer, 4–6, 7–9, and more than 9 days were 87.0% (100/115), 92.8% (256/276), 90.3% (213/236), and 88.0% (110/125), respectively. The incidence of pleural effusion was significantly different from the duration of fever prior to admission (P=0.004). Despite differences in the duration of fever before admission, there was little change in the proportions of the lobar consolidation (P=0.14; Figure 3). And the levels of inflammatory markers, such as LDH, IL-2R, and CRP, consistently rose with the grade of lobar consolidation (Figure 4). The LDH, IL-2R, and CRP levels of in those with 4 lobes of consolidation were much higher than in those with 0 lobes of consolidation or 1 lobe of consolidation (P<0.001).
Clinical predictive performance of key CT features
In the Kaplan-Meier analysis, the risk of fever duration after admission (P=0.01–0.02) and the length of stay (P<0.001) increased significantly with lobar consolidation (Figure 5). After adjustments for sex, age, and pleural effusion, Cox proportional-hazard models still demonstrated that increased lobar consolidation was positively associated with the duration of fever after admission, with the longest time observed for 4 lobar consolidations, followed by 3 lobar consolidations, 2 lobar consolidations, and 1 lobar consolidation (Table 4). Similarly, the same trend was observed in the length of hospital stay. The increasing number of lobar consolidations led to gradually increasing costs, including in hospitalization charges, laboratory tests, imaging costs, and medication expenses (Table 5).
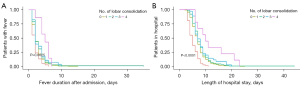
Table 4
| Number of lobes | Endpoint 1: duration of fever after admission (days) | Endpoint 2: length of hospital stay (days) | |||
|---|---|---|---|---|---|
| Hazard ratioa (95% CI) | P valuea | Hazard ratioa (95% CI) | P valuea | ||
| 0 | Ref | – | Ref | – | |
| 1 | 1.55 (1.10–2.18) | 0.012 | 2.24 (1.73–2.89) | <0.001 | |
| 2 | 1.65 (1.13–2.42) | 0.01 | 2.56 (1.91–3.43) | <0.001 | |
| 3 | 1.82 (1.11–2.98) | 0.02 | 2.87 (1.90–4.32) | <0.001 | |
| 4 | 2.87 (1.25–6.61) | 0.01 | 4.12 (2.01–8.46) | <0.001 | |
All hazard ratio calculations were made using ≤75th percentile value data as a reference. a, adjusted for age, sex, and pleural effusion. MPP, Mycoplasma pneumoniae pneumonia; CT, computed tomography; CI, confidence interval.
Table 5
| Costs | Number of lobes | ||||
|---|---|---|---|---|---|
| 0 (n=73) | 1 (n=490) | 2 (n=145) | ≥3 (n=44) | P value | |
| Hospital charges, $ | 1,022.6 (846.7–1,133.3) | 1,535.8 (1,110.5–1,964.5) | 1,675.5 (1,135.9–2,138.1) | 1,875.5 (1,257.1–2,633.0) | <0.001 |
| Laboratory tests, $ | 414.3 (333.4–542.5) | 627 (396.5–816.6) | 611 (400.9–816.6) | 646.8 (423.2–865.0) | <0.001 |
| Imaging costs, $ | 67.7 (38.5–84.2) | 107.7 (64.6–128.3) | 104.9 (66.2–127.8) | 120.0 (78.5–131.9) | <0.001 |
| Medication, $ | 206.2 (151–282.4) | 345.5 (238.2–488.3) | 389.4 (259.2–545.7) | 429.4 (282.6–704.6) | <0.001 |
Data are presented as the median (25th−75th percentile). CT, computed tomography.
Discussion
In our large-sample study, lung consolidation was a stable and reliable CT feature for assessing disease severity in patients with MPP. Lobar consolidation was independently associated with a higher risk of SMPP, higher levels of inflammatory markers, longer fever duration and length of stay, and higher healthcare costs. These results demonstrated that an increased number of lobar consolidations could predict the severity of MPP and the clinical progression of MPP in patients at an early stage.
Radiographic manifestations of MPP are diverse and include bronchial wall thickening; reticulonodular, segmental and lobar consolidations; atelectasis; hilar lymphadenopathy; and pleural effusion. Of these, consolidation, which presents as a uniform increase in lung parenchymal attenuation that obscures the margins of vessels and airway walls, is more common in MPP than in other CAPs on chest X-rays or chest CT, appearing in 33–79% of the cases (17). In this study, consolidation was the second most common imaging feature, and the incidence of consolidation was as high as 87.0% in patients with a fever duration ≤3 days and 88.0% for those with a fever duration >9 days. Therefore, consolidation was the common and stable CT feature in the large MPP study. The consolidation rate was relatively high in the early stages of infection and did not increase significantly with an increase in fever duration before admission, which may be relevant to type IV hypersensitivity (18). Type IV hypersensitivity, called delayed hypersensitivity, is a pathological manifestation mediated by immune cells. Patients with MPP may be allergic to mycoplasma antigen or antibody complexes, which sensitize T cells. When exposed to the antigen again, the T cells become killer cells or release lymphokines, causing a severe immune response. Possible hypotheses for the mechanism and etiology of fulminant MPP reported in the literature are as follows: (I) a hyperimmune response originating in the lung due to recurrent childhood Mycoplasma pneumoniae infections; and (II) loss of the ability to eradicate Mycoplasma pneumoniae from the lung in primary infection resulting in longer-lasting Mycoplasma pneumoniae infection in the lung, which may cause a hyperimmune response (18,19).
Furthermore, we found that an increased number of lobar consolidations were associated with higher odds of SMPP. Previous literature reported that patients with consolidation were more prone to have hypoxia, tachypnoea, tachycardia, and extrapulmonary manifestations, suggesting that pneumonia with consolidation in children was more severe than that without consolidation on chest X-ray (17). Additionally, our research showed that pleural effusion had the greatest weight in the prediction of SMPP. However, only 15.8% of patients developed pleural effusion (119/752), which was much lower than the rate of consolidation in children with MPP. Moreover, the incidence of pleural effusion increased significantly with an increase in fever duration before admission. Therefore, the stability of pleural effusion may be inferior to consolidation. Finally, we concluded that lobar consolidation was the stable and high-risk feature for predicting SMPP. According to management guidelines for severe pneumonia, multilobar infiltration is a secondary diagnostic criterion (15). However, lung infiltration includes multiple features, and consolidation is only one of these. In contrast to previous vague assessments of multilobar infiltration and large area or multilobar consolidation, we demonstrated the lobar consolidation to be a stable prediction feature. We also successfully conducted a quantitative evaluation of lobar consolidation and obtained a predictive model for SMPP using various CT features.
Inflammatory cytokines are involved in the immunopathogenesis of Mycoplasma pneumoniae infection (5,20,21). Our study found a positive correlation between lobar consolidation and LDH, IL-2R, and CRP levels in children with MPP, which was consistent with the findings of previous studies (21,22). Mycoplasma pneumoniae attaches to the ciliated epithelial cells on the respiratory tract through the P1 protein, exerting cytotoxicity by expression of community-acquired respiratory distress syndrome and production of hydrogen peroxide, thereby activating host immunity, including macrophages, mast cells, neutrophils, and natural killer cells, as well as T and B lymphocytes and humoral immune responses (23). Cell-mediated immunological responses play an essential role in the development of MPP. In SMPP, the immune response is exaggerated, and interleukin levels are elevated, resulting in diffuse alveolar damage with fibrinous exudates within the alveolar lumens, which is shown as consolidation on CT (6). Hence, the positive correlation between lobar consolidation and the level of inflammatory cytokines further indicated that lobar consolidation was a reliable CT feature for assessing the severity of MPP.
Some studies have previously investigated the association of multilobar involvement with the clinical course of MPP (13,24). Considering the weight of pleural effusion, we adjusted for its effects in our Cox analysis and found that patients with more lobar consolidations still experienced longer fever durations, longer lengths of hospital stay, and higher medical costs. Thus, the number of consolidated lung lobes can be used to quantitatively assess the clinical course of MPP and is superior to the previous use of multilobar involvement to precisely predict the clinical course, thereby guiding rational clinical treatments with major clinical significance.
Clinicians are cautious about the use of CT imaging in children because of the potential risks of radiation exposure. In this study, we used low-dose CT to assess MPP. According to the scanning parameters, patients weighing less than 20 kg absorb about 0.4–0.8 mSv of radiation, equivalent to the dose of 4 to 8 chest radiographs, and those weighing 20–60 kg absorb about 0.7–1.6 mSv, equal to the dose of 7 to 16 chest radiographs (25,26). Based on the previous evidence, the radiation dose of 30–90 mSv for children may increase the risk of cancer (27). According to the risk estimate of 10 mSv exposure per million patients, a 5-year-old child has twice the lifetime risk of lung cancer as does a 50-year-old adult (28). In our study, the radiation doses received by all patients were 0.4–1.6 mSv, well below 30–90 mSv, and the children were older than 5 years old. Therefore, we suspect that the low-dose CT applied in our study was safe in children. In contrast to the 33–79% incidence of consolidation currently reported, our results showed that the proportion of patients with consolidation was up to 90.3%, which was attributed to the superiority of CT over X-rays in demonstrating lesion patterns and lung anatomy. Large-area or entire-lobe consolidation can be clearly observed on chest X-ray and CT, while patchy consolidation indicative of bronchopneumonia on CT may manifest as a nonconsolidated feature on chest X-ray. Additionally, we quantitatively evaluated lobar consolidation, which apparently cannot be quantified by chest X-ray (29). CT is recommended for assessment when patients are unresponsive to therapy, have severe complications on chest X-ray, or need to rule out HIV infection and tuberculosis (11). Hence, there is a tradeoff between satisfying diagnostic image quality and the feasibility of using CT radiation doses. Therefore, low-dose CT is recommended for children with MPP who do not respond well or require a differential diagnosis.
Our study has a few limitations. First, this study was conducted retrospectively, so the analysis was limited to the available medical records. There was inevitable selection bias in the process of data collection, although we established the reviewing expert committee to re-evaluate the clinical information of patients. Second, we could not obtain the patients’ lung pathological specimens; as a result, we could not analyze the correlation between imaging and pathology. Considering the repeatability and operability of this study, CT is a noninvasive examination technique that can best reflect the actual pathological condition in children with MPP. Third, the imaging findings in this study were highly influenced by the CT scanning time. All participants in this study underwent CT scans within 24 hours of admission to reduce the bias as much as possible. Meanwhile, there was no significant difference in the statistical results of chest CT examination time between the two groups. However, we cannot guarantee that the CT images of all patients were obtained at the same disease stage. Despite these limitations, associations among the evaluated CT-based variables were identified and appear convincing.
Conclusions
Our findings showed that lobar consolidation is a stable and reliable CT feature for evaluating the severity of MPP. Quantitative analysis of lobar consolidation can comprehensively and accurately assess and predict MPP progression. Low-dose CT is recommended for children with complex and severe MPP.
Acknowledgments
Funding: This investigation was supported by the National Natural Science Foundation of China (Nos. 81873947, 82172138 and 81874265), the Shanghai Natural Science Foundation of China (No. 2017YQ033), the Shanghai Talent Development Foundation (No. 2018003), and the Industrial Collaborated Research Foundation of Shanghai Jiao Tong University (No. YG2017MS75).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-508/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-508/coif). CG reports that this work was supported by the National Natural Science Foundation of China (Nos. 81873947 and 82172138). LH reports that this work was supported by the National Natural Science Foundation of China (No. 81874265), the Shanghai Natural Science Foundation of China (No. 2017YQ033), the Shanghai Talent Development Foundation (No. 2018003), and the Industrial Collaborated Research Foundation of Shanghai Jiao Tong University (No. YG2017MS75). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Clinical Research of Xinhua Hospital (No. XHEC-D-2021-154), and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jain S, Williams DJ, Arnold SR, Ampofo K, Bramley AM, Reed C, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med 2015;372:835-45. [Crossref] [PubMed]
- Gao LW, Yin J, Hu YH, Liu XY, Feng XL, He JX, Liu J, Guo Y, Xu BP, Shen KL. The epidemiology of paediatric Mycoplasma pneumoniae pneumonia in North China: 2006 to 2016. Epidemiol Infect 2019;147:e192. [Crossref] [PubMed]
- Kutty PK, Jain S, Taylor TH, Bramley AM, Diaz MH, Ampofo K, Arnold SR, Williams DJ, Edwards KM, McCullers JA, Pavia AT, Winchell JM, Schrag SJ, Hicks LA. Mycoplasma pneumoniae Among Children Hospitalized With Community-acquired Pneumonia. Clin Infect Dis 2019;68:5-12. [PubMed]
- Moynihan KM, Barlow A, Nourse C, Heney C, Schlebusch S, Schlapbach LJ. Severe Mycoplasma Pneumoniae Infection in Children Admitted to Pediatric Intensive Care. Pediatr Infect Dis J 2018;37:e336-e338. [Crossref] [PubMed]
- Xu XF, Li XJ, Liu JL, Wu L, Chen ZM. Serum cytokine profile contributes to discriminating M. pneumoniae pneumonia in children. Cytokine 2016;86:73-8. [Crossref] [PubMed]
- Tanaka H. Correlation between Radiological and Pathological Findings in Patients with Mycoplasma pneumoniae Pneumonia. Front Microbiol 2016;7:695. [Crossref] [PubMed]
- Saraya T, Watanabe T, Tsukahara Y, Ohkuma K, Ishii H, Kimura H, Yan K, Goto H, Takizawa H. The Correlation between Chest X-ray Scores and the Clinical Findings in Children and Adults with Mycoplasma pneumoniae Pneumonia. Intern Med 2017;56:2845-9. [Crossref] [PubMed]
- Reittner P, Müller NL, Heyneman L, Johkoh T, Park JS, Lee KS, Honda O, Tomiyama N. Mycoplasma pneumoniae pneumonia: radiographic and high-resolution CT features in 28 patients. AJR Am J Roentgenol 2000;174:37-41. [Crossref] [PubMed]
- Lee I, Kim TS, Yoon HK. Mycoplasma pneumoniae pneumonia: CT features in 16 patients. Eur Radiol 2006;16:719-25. [Crossref] [PubMed]
- Miller JM, Binnicker MJ, Campbell S, Carroll KC, Chapin KC, Gilligan PH, Gonzalez MD, Jerris RC, Kehl SC, Patel R, Pritt BS, Richter SS, Robinson-Dunn B, Schwartzman JD, Snyder JW, Telford S 3rd, Theel ES, Thomson RB Jr, Weinstein MP, Yao JD. A Guide to Utilization of the Microbiology Laboratory for Diagnosis of Infectious Diseases: 2018 Update by the Infectious Diseases Society of America and the American Society for Microbiology. Clin Infect Dis 2018;67:e1-e94. [Crossref] [PubMed]
- Andronikou S, Goussard P, Sorantin E. Computed tomography in children with community-acquired pneumonia. Pediatr Radiol 2017;47:1431-40. [Crossref] [PubMed]
- Miyashita N, Sugiu T, Kawai Y, Oda K, Yamaguchi T, Ouchi K, Kobashi Y, Oka M. Radiographic features of Mycoplasma pneumoniae pneumonia: differential diagnosis and performance timing. BMC Med Imaging 2009;9:7. [Crossref] [PubMed]
- Guo Q, Li HY, Zhou YP, Li M, Chen XK, Peng HL, Yu HQ, Liang LH, Zhao QZ, Jiang M. Associations of radiological features in Mycoplasma pneumoniae pneumonia. Arch Med Sci 2014;10:725-32. [Crossref] [PubMed]
- Gong L, Zhang CL, Zhen Q. Analysis of clinical value of CT in the diagnosis of pediatric pneumonia and mycoplasma pneumonia. Exp Ther Med 2016;11:1271-4. [Crossref] [PubMed]
- Bradley JS, Byington CL, Shah SS, Alverson B, Carter ER, Harrison C, Kaplan SL, Mace SE, McCracken GH Jr, Moore MR, St Peter SD, Stockwell JA, Swanson JT. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis 2011;53:e25-76. [Crossref] [PubMed]
- Tamura A, Matsubara K, Tanaka T, Nigami H, Yura K, Fukaya T. Methylprednisolone pulse therapy for refractory Mycoplasma pneumoniae pneumonia in children. J Infect 2008;57:223-8. [Crossref] [PubMed]
- Cho YJ, Han MS, Kim WS, Choi EH, Choi YH, Yun KW, Lee S, Cheon JE, Kim IO, Lee HJ. Correlation between chest radiographic findings and clinical features in hospitalized children with Mycoplasma pneumoniae pneumonia. PLoS One 2019;14:e0219463. [Crossref] [PubMed]
- Izumikawa K. Clinical Features of Severe or Fatal Mycoplasma pneumoniae Pneumonia. Front Microbiol 2016;7:800. [Crossref] [PubMed]
- Nisar N, Guleria R, Kumar S, Chand Chawla T, Ranjan Biswas N. Mycoplasma pneumoniae and its role in asthma. Postgrad Med J 2007;83:100-4. [Crossref] [PubMed]
- Chaudhry R, Ghosh A, Chandolia A. Pathogenesis of Mycoplasma pneumoniae: An update. Indian J Med Microbiol 2016;34:7-16. [Crossref] [PubMed]
- Cheng S, Lin J, Zheng X, Yan L, Zhang Y, Zeng Q, Tian D, Fu Z, Dai J. Development and validation of a simple-to-use nomogram for predicting refractory Mycoplasma pneumoniae pneumonia in children. Pediatr Pulmonol 2020;55:968-74. [Crossref] [PubMed]
- Zhao JL, Wang X, Wang YS. Relationships between Th1/Th2 cytokine profiles and chest radiographic manifestations in childhood Mycoplasma pneumoniae pneumonia. Ther Clin Risk Manag 2016;12:1683-92. [Crossref] [PubMed]
- Waites KB, Xiao L, Liu Y, Balish MF, Atkinson TP. Mycoplasma pneumoniae from the Respiratory Tract and Beyond. Clin Microbiol Rev 2017;30:747-809. [Crossref] [PubMed]
- Yoon IA, Hong KB, Lee HJ, Yun KW, Park JY, Choi YH, Kim WS, Lee H, Eun BW, Ahn YM, Cho EY, Cho HJ, Choi EH. Radiologic findings as a determinant and no effect of macrolide resistance on clinical course of Mycoplasma pneumoniae pneumonia. BMC Infect Dis 2017;17:402. [Crossref] [PubMed]
- Nievelstein RA, van Dam IM, van der Molen AJ. Multidetector CT in children: current concepts and dose reduction strategies. Pediatr Radiol 2010;40:1324-44. [Crossref] [PubMed]
- Greffier J, Hoballah A, Sadate A, de Oliveira F, Claret PG, de Forges H, Loubet P, Mauboussin JM, Hamard A, Beregi JP, Frandon J. Ultra-low-dose chest CT performance for the detection of viral pneumonia patterns during the COVID-19 outbreak period: a monocentric experience. Quant Imaging Med Surg 2021;11:3190-9. [Crossref] [PubMed]
- Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med 2007;357:2277-84. [Crossref] [PubMed]
- Research NRCU. Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII, Phase I, Letter Report (1998). Washington (DC): National Academies Press (US), 1998.
- Kotok D, Yang L, Evankovich JW, Bain W, Dunlap DG, Shah F, Zhang Y, Manatakis DV, Benos PV, Barbash IJ, Rapport SF, Lee JS, Morris A, McVerry BJ, Kitsios GD. The evolution of radiographic edema in ARDS and its association with clinical outcomes: A prospective cohort study in adult patients. J Crit Care 2020;56:222-8. [Crossref] [PubMed]



