
A rare giant cardiac lymphangioma in adults
Introduction
Cardiac lymphangioma is a rare benign cardiac tumour and most of them occur during childhood. Recent articles reviewed the current literature of 39 cases of primary cardiac and pericardial lymphangioma with detailed analysis of clinical and imaging findings of cardiac lymphangioma (1-3). Of these 39 cases, coronary arteries were involved in only 11 cases with the right coronary artery and the first obtuse marginal branch of left circumflex encroached in 10 cases and one case, respectively (3). Details of imaging appearances were reported in 23 cases of cardiac lymphangioma including chest X-ray (n=12), echocardiography (n=22), computed tomography (CT) (n=16) and magnetic resonance imaging (MRI) (n=16) findings (3). To the best of our knowledge, there are no reports of comprehensive imaging analysis with inclusion of all imaging modalities in the diagnosis of cardiac lymphangioma. A comprehensive correlation of imaging findings with clinical data and pathology is of great significance in assisting clinical diagnosis and decision-making of primary cardiac lymphangioma. Herein, we reported a case of asymptomatic giant cystic lymphangioma adjacent to the lateral posterior wall of the left ventricle with 4 years of follow-up results. This case highlights the benign imaging appearances of cardiac lymphangioma with results correlated with the pathological findings.
Case presentation
A 50-year-old male without any clinical symptoms with a cardiac mass detected on physical examination in the regional hospital was referred to our cardiac center for further diagnosis. Upon physical examination, his blood pressure was 100/60 mmHg, and heart rate was 90 beats/min. The survey data of the patient showed that hemoglobin, platelet and erythrocyte sedimentation rate were normal. Cardiac examination revealed a regular rhythm without abnormal murmurs. No cyanosis, or edema of the arms, hands, legs, or feet was noted. Posteroanterior and lateral chest radiographs (Figure 1A,1B) showed a round, high-density lesion in the cardiac shadow with an elevated left diaphragm. The patient would like to know the nature of the mass after finding abnormality on chest radiographs, thus, additional imaging examinations were requested to determine the nature of the lesion. Echocardiography showed a space-occupying lesion with a mixed echo appearance in the left atrioventricular sulcus pericardium (from the inferior wall of the left ventricle, the posterior wall and the left atrioventricular ring to the posterior wall of the left atrium) (Figure 1C-1E). The margin of the lesion was complete and in contact with the pericardial visceral layer. The size of the lesion was about 12.2×6.6 cm2. The myocardium was not invaded by the mass without obvious restrictions in the relaxation of the ventricular wall. Since ultrasound examination could not be diagnostic, CT scan was requested to clarify the imaging appearances of the cardiac lesion.
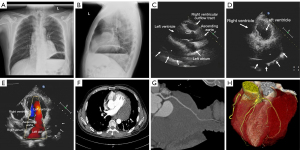
On the contrast-enhanced CT images (Figure 1F), a normal peripheral fat space was observed between the mass and the myocardium. The mass surrounded the left circumflex artery, with a smooth arterial wall and the lumen patent without any stenosis (Figure 1G,1H). Because of the static imaging of contrast-enhanced CT, it is difficult to determine the internal components of the mass, cardiac magnetic resonance (CMR) examination including multi-sequence and multi-parameter imaging was requested to determine the nature of the mass due to its superior advantages in demonstrating soft tissue characteristics through analysis of signal intensity changes within both normal and abnormal structures.
CMR showed a space-occupying lesion in the posterior mediastinum near the pericardium. The boundary of the mass was distinct, and cystic component was detected in the mass. The interface of the mass was clear without thickening or nodules in the cystic wall. The irregular lymphatic vessels in the tumour contained a clear liquid. The shape and size of the cysts altered with fluctuating cardiac pressure, and moved regularly with the blood in different cardiac stages. The lesion showed iso-intensity in T1 weighted image (T1WI) and high signal intensity in T2 weighted image (T2WI) (Figure 2A,2B). However, no late gadolinium enhancement (LGE) was observed after contrast enhancement imaging (Figure 2C,2D). The cine sequence showed an unrestricted movement of the left ventricle. Moreover, there was no pericardial effusion, and the thickness of the pericardium was uniform at <2 mm without any nodules. Positron emission tomography (PET) imaging was considered to confirm whether there were any similar lesions or metastases in other body parts. PET (Figure 2E) scan displayed a large soft tissue mass in the pericardium, without increased glucose metabolism.
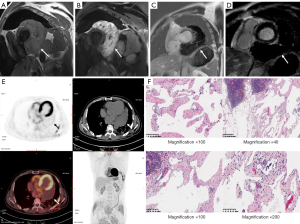
Exploratory thoracotomy revealed a large, lobulated dark red, soft texture and 11×10.5×10 cm3 tumour in the pericardial cavity. It was closely attached to the wide base of the left ventricle and left atrium. A communicating vessel was noticed between the left circumflex branch and the mass, thus complete resection of the tumour could not be performed. The patient only received exploratory thoracotomy, and a small amount of tissues were taken for pathological biopsy, without massive resection. The pathological tissues were excised during the operation, and the postoperative histopathological report revealed cystic lymphangioma (Figure 2F). The patient was followed up for 4 years, and the mass had not changed significantly in diameter.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Lymphangioma is a benign tumour originating from mesenchymal tissue and formed by the development and proliferation of primitive lymphatics. Cystic lymphangioma is usually confined to the head and neck, and hence, lymphangioma in the heart is extremely rare (1-5). Of 39 cases of cardiac lymphangioma reviewed in the literature, 29 were found to be situated on the pericardium (3). In most cases, the compression of adjacent organs could change accordingly. The type of symptoms depends on the scope and degree of involvement, including adverse cardiovascular events, such as syncope or palpitation, arrhythmia or congestive heart failure, while only 8 cases were asymptomatic (3). This rare asymptomatic case was introduced to emphasize the atypical clinical symptoms and the advantages of imaging examination in the localization of fine tumour anatomy and the differentiation of benign and malignant tumours before the diagnosis of the pathological biopsy. Our case report presents two advantages over other reports. First, a comprehensive imaging examination was conducted in this case showing imaging appearances from general chest radiography to ultrasound, CT, MRI and PET, with findings confirmed pathologically. In particular, the PET imaging appearances confirmed the benignity of the tumour which add value to the diagnosis of this rare cardiac tumour. Second, the patient was followed up for 4 years, and no significant change was detected in the tumour size. These findings added valuable information to the existing literature, thus assisting the clinicians in making an accurate diagnosis when encountering similar scenarios.
In this case, the mass was adjacent to the left ventricular wall and protruded in the mediastinum. The cystic mass was close to the left ventricle, and no abnormality was detected in the cardiac structures, similar to previous reports (1-5). Cystic lymphangioma has the potential risk of recurrence, especially in the case of incomplete resection. In this case, the detection of cardiac lymphangioma mainly relied on imaging examinations. The origin, texture, boundary, size and tissue surrounding the mass were distinct. Based on cardiac function, we determined that the mass is a benign component as observed on CT, MRI and PET images. CMR provides the tissue characteristics of the mass and its correlation with cardiac motion, which is crucial for locating the origin of the tumour (5). Although the lesion was not removed completely during surgery, the patient did not develop any adverse cardiovascular events and systemic adverse conditions of pericardial tamponade or cardiac insufficiency in the 4-year follow-up, indicating the benignity of the tumour.
In conclusion, we reported a rare giant cardiac lymphangioma in an adult patient with imaging appearances consistent with a benign tumour; the diagnosis was confirmed pathologically. The tumour was removed partially, and the patient was followed up for 4 years; however, no significant change was detected in the tumour size. The finding of left circumflex artery being enveloped by the lymphangioma in this case provides further value to the involvement of coronary arteries in primary cardiac lymphangioma as only one-third of coronary arteries are shown to be involved in the literature. This case report adds valuable information to the current literature due to the rare occurrence of cardiac lymphangioma and assists in timely diagnosis when clinicians encounter similar case scenarios.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (No. 82071880).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-794/coif). ZS serves as an unpaid associate editor of Quantitative Imaging in Medicine and Surgery. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Diao WJ, Shi C, Liu G, Liu XG, Li HH, Meng JJ, Shi Y, Chang MM, Liu YY. The diagnosis and treatment of cardiac lymphangioma: A case report and literature review. Medicine (Baltimore) 2019;98:e14000. [Crossref] [PubMed]
- Cailleba L, Labrousse L, Marty M, Montaudon M, Gerbaud E. Pericardial cystic lymphangioma. Eur Heart J Cardiovasc Imaging 2013;14:246. [Crossref] [PubMed]
- Pichler Sekulic S, Sekulic M. Primary cardiac and pericardial lymphangiomas: clinical, radiologic, and pathologic characterization derived from an institutional series and review of the literature. Virchows Arch 2022;480:1211-21. [Crossref] [PubMed]
- Kim SJ, Shin ES, Kim SW, Shin JK, Cheong JP, Kim YM, Lee SG. A case of cardiac lymphangioma presenting as a cystic mass in the right atrium. Yonsei Med J 2007;48:1043-7. [Crossref] [PubMed]
- Kaji T, Takamatsu H, Noguchi H, Tahara H, Matsuda H, Nomura Y, Machigashira S, Watanabe S, Yoshioka T. Cardiac lymphangioma: case report and review of the literature. J Pediatr Surg 2002;37:E32. [Crossref] [PubMed]


