
Predictors of hyperperfusion syndrome after stent implantation in symptomatic intracranial atherosclerotic stenosis
Introduction
Intracranial atherosclerotic stenosis (ICAS) is an important cause of ischemic stroke, accounting for approximately 10–20% of ischemic stroke in western populations and 30–50% in Asian populations (1). Despite aggressive medical management, the 1-year rate of recurrent stroke remained at 12.6% in the SAMMPRIS (Stenting and Aggressive Medical Management for the Prevention of Recurrent Stroke in Intracranial Stenosis) trial (2). For patients with severe ICAS refractory to medical treatment, stent implantation has been demonstrated to be an effective therapy for reducing the risk of stroke recurrence (3,4). However, hyperperfusion syndrome (HPS), an uncommon but serious perioperative complication after stent implantation, may affect the safety and efficacy of stents (5). The symptoms of HPS after cerebrovascular recanalization are characterized by headache, focal neurological deficits, or seizures, and the symptoms persistently deteriorate when accompanied by intracranial hemorrhage (ICH) (6). The rate of HPS complicated with ICH following intracranial arterial endovascular therapy is approximately 2–3.37%, but the mortality can be as high as 50% (7,8). Therefore, identifying predictors of HPS is particularly crucial for its prevention following intracranial stent implantation.
Previous studies have shown that excessive preprocedural hypoperfusion is closely associated with HPS after carotid artery stenting (CAS) (9,10). Some neuroimaging tools can be used to evaluate cerebral perfusion and play diagnostic and predictive roles in ischemic stroke (11,12). Computed tomography perfusion (CTP) can accurately assess cerebral perfusion and has been reported to predict the HPS after CAS (13). However, the predictive value of CTP for HPS after stenting in ICAS patients remains unknown. RAPID (iSchemia View, Denver, CO, USA) is a quantitative automated CTP post-processing software for assessing cerebral hypoperfusion, and its effectiveness has been confirmed by previous studies (14,15). The perfusion assessed using CTP is more suitable for anterior circulation than posterior circulation due to physiologic hemodynamic delay and the tendency for anatomical variation in the posterior circulation (16). Therefore, we conducted this study to investigate the predictors of HPS after stent implantation in patients with symptomatic severe anterior circulation ICAS using the RAPID software. We present the following article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-682/rc).
Methods
Study population
In this retrospective case-control study, we collected data from consecutive patients who underwent stent implantation due to symptomatic anterior circulation ICAS in a high-volume stroke center from June 2012 to September 2019. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Ethics Committee of Beijing Tiantan Hospital (No. KY2013-013-01), and individual consent for this retrospective analysis was waived. The baseline data, including age, sex, atherosclerotic risk factors, qualifying events, and medical history, were collected by a central adjudication committee consisting of neurologists, interventionists, and radiologists who were blinded to the study design. The conduct of the study was supervised by an independent data and safety monitoring board.
The patient inclusion criteria were as follows: (I) age 18–85 years old; (II) 70–99% stenosis of the responsible arteries, including intracranial internal carotid artery (ICA) and the M1 segment of the middle cerebral artery (MCA), assessed using the WASID (warfarin-aspirin symptomatic intracranial disease) method (17); (III) transient ischemic attack (TIA) or non-disabling ischemic stroke attributed to the territory of the target lesion, where ischemic stroke was defined as a new focal neurologic deficit lasting for 24 h or longer or lasting less than 24 h with new infarction on imaging, and TIA was defined as acute onset of a focal neurologic deficit lasting less than 24 h without new infarction on imaging; and (IV) resistance to medical treatment (dual antiplatelet and risk factors management including blood pressure, low-density lipoprotein cholesterol, diabetes, smoking and weight).
Patients were excluded if they (I) had experienced acute cerebral infarction within 2 weeks of the study; (II) had non-atherosclerotic stenosis, including moyamoya disease, muscle fiber dysplasia, or artery dissection; (III) had a potential cardiac embolism; (IV) were complicated with an intracranial tumor and cerebral arteriovenous malformation; (V) had a tandem lesion with extracranial stenosis or another intracranial large artery stenosis; and/or (VI) lacked imaging data.
Processing of CTP data
All the included patients received a CTP examination before the procedure. CTP images were acquired using a 256-slice axial computed tomography (CT) scanner (GE Revolution CT; GE Healthcare, Chicago, IL, USA). Contrast agent, 50 mL (Omnipaque, 350 mg I/mL; GE Healthcare, Shanghai, CN), was injected into the antecubital vein at a rate of 5 mL/s using an automated injector (Ulrich Injection System; Ulrich GMBH & Co. KG, Ulm, Germany). After a 5 s delay, CTP was performed with the following acquisition parameters: 80 kV tube voltage, 150 mA, 5 mm slice thickness, 256×0.625 mm collimation, 0.5 s rotation time, 2.0 s cycle time, 25-cm field of view (FOV), 512×512 image matrix size, and 32 slices. A total of 512 slices were obtained with a 160 mm scan length and scan time of about 40 s. CTP data were postprocessed with the automated RAPID software (iSchemia View). Cerebral perfusion was estimated from the volume of tissue for which there was delayed arrival of an injected tracer agent (Tmax (time-to-maximum of the residue function)). Cerebral hypoperfusion was defined as voxels with Tmax >6 s and Tmax >4 s. These thresholds were chosen based on a previous study which showed that Tmax >6 s and Tmax >4 s were reliable estimates of the volume of the ischemic penumbra in ischemic stroke patients (18). The core infarct volume was defined as a volume of cerebral blood flow (CBF) less than 30%.
Interventional procedure and device selection
All interventional procedures were performed by experienced neurointerventionists. Patients were given either local or general anesthesia according to preoperative assessment. A bolus dose (75 U/kg) of intravenous heparin was administered after the right femoral artery puncture, followed by half the dose 1 h later. A guide catheter was advanced into the ICA. A 0.014-inch microwire was carefully manipulated across the lesion. Device selection was based on the access characteristics and lesion features (Mori types) as well as operator experience. For smooth, local, or Mori A lesions, an apollo balloon-mounted stent was preferred. For long, tortuous arterial access, or Mori C lesions, a balloon dilation plus a self-expanding stent (Gateway balloon plus Wingspan stent system; Stryker, Maple Grove, MN, USA) was preferred.
Periprocedural management
All patients fasted for at least 12 h before the procedure. Noncontrast head CT was obtained to detect ICH after the procedure. Patients were given antihypertensive medication when their blood pressure exceeded 140/90 mmHg to lower the risk of hyperperfusion injury. The patient’s electrocardiogram, vital signs, and neurological functions were strictly monitored at least 24 h after the procedure.
Medical treatment
Before the procedure, all patients received dual-antiplatelet therapy (DAPT) (aspirin 100 mg/day plus clopidogrel 75 mg/day) for at least 5 days or a loading dose of DAPT (300 mg aspirin plus 300 mg clopidogrel). All patients received standard DAPT for 90 days after stenting and suspended the use of either aspirin or clopidogrel 90 days later based on the resistance testing for antiplatelet drugs. Risk factors management included blood pressure lowering than 140 mmHg (130 mmHg in patients with diabetes mellitus), low-density lipoprotein lowering than 1.81 mmol/L, cessation of smoking, and lifestyle improvement.
HPS definition
According to whether HPS occurred after the procedure, all patients were divided into the HPS group and the non-HPS group. Patients who satisfied either of the following 2 criteria within 30 days after the procedure were defined as HPS: (I) CT confirmed intracerebral hemorrhage or subarachnoid hemorrhage with or without clinical symptoms and ruled out technology-related hemorrhage; or (II) the patient presented with an ipsilateral new-onset headache, seizure, focal neurological deficit, altered mental status, or decreased level of consciousness, but these symptoms arisen from new ischemic stroke or other metabolic or pharmacological causes was ruled out, and the post-procedural peak blood flow velocity of normal vessels in the distal ipsilateral MCA demonstrated by transcranial Doppler sonography (TCD) increased by more than 100% compared with the preoperative value.
We screened for patients with HPS. First, the post-procedural head CT was screened to determine the presence or absence of ICH. ICH due to vessel perforation caused by a guidewire, vessel rupture after balloon dilatation, or other contrast media extravasation during the procedure was identified as a technology-related hemorrhage. Second, patients with the symptoms of focal neurological deficit were screened for ischemic stroke by post-procedural magnetic resonance imaging (MRI). Third, patients with any symptoms suspected to be HPS were screened by TCD. The diagnosis of HPS was ultimately determined based on clinical manifestations, head CT, MRI, and TCD.
Statistical analysis
Continuous variables are presented as median (interquartile range) or the mean ± standard deviation and compared using the Student’s t-test for variables with normal distribution or the Mann-Whitney U test for variables with nonnormal distribution. The Kolmogorov-Smirnov test was used to test distribution normality. Categorical variables were presented as a number (percentage) and compared using the χ2 test or Fisher exact test. The predictive values of Tmax >4 s and Tmax >6 s in HPS were tested using receiver operating characteristic (ROC) analysis. A 2-tailed P value less than 0.05 was considered statistically significant. Statistical analysis was performed using SPSS 23.0 (IBM Corp., Armonk, NY, USA).
Results
Baseline characteristics
Among a total of 1,960 screened patients, 170 cases (mean age, 57.0±8.63 years; 70.6% men) were eligible for our study. Figure 1 shows the flow chart of participant selection. Out of those 170 patients, 125 (73.5%) had hypertension, 76 (44.7%) had diabetes mellitus, 86 (50.6%) had hyperlipidemia, and 85 (50%) were smokers. The responsible lesions were ICA in 64 (37.6%) patients and MCA in 106 (62.4%) patients. The median time from the qualifying event to CTP was 55 [interquartile range (IQR), 31.8–170.5] days, and the median time from CTP to endovascular treatment was 4.5 (IQR, 2.5–8.7) days. The medians of arterial stenosis, residual stenosis, and degree of stenosis improvement were 80% (IQR, 70–90%), 10% (IQR, 5–15%), and 70% (IQR, 60–80%), respectively. Among all eligible patients, TCD was performed on 122 (71.76%).
Incidence of HPS
Among 170 patients, the post-procedural head CT showed that 3 had ICH that was determined to be HPS without technology-related hemorrhage. Focal neurological deficits symptoms occurred in 4 patients after the operation. One patient had aphasia, 1 had alalia, 1 had numbness of limbs, and 1 had weakness of limbs. Postprocedural MRI confirmed that these 4 patients had a new ischemic stroke. A total of 32 patients presented with headaches, and 1 patient was identified as having HPS by TCD. Two patients presented with altered mental status, 1 with dysphoria and 1 with delirium, and were determined to have HPS by TCD. No patient presented with seizures after the procedure. A total of 31 patients for whom peak blood flow velocity increased by more than 100% had no clinical manifestations. The remaining 48 patients without TCD had no clinical manifestations, and these patients were classified as non-HPS.
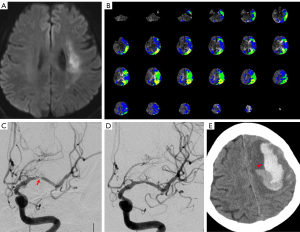
Finally, a total of 6 (3.53%) cases developed HPS after the procedure, including 5 males and 1 female with an average age of 59.67±8.24 years. Three of the responsible arteries were located in ICA and 3 were in MCA (Table 1). All of their symptoms developed within 24 h after the procedure. Among the 6 patients with HPS, 3 patients presented with ICH, 1 with dysphoria, 1 with delirium, and 1 with a headache (Table 2). Among the 3 ICH patients, 2 died, and 1 had a severe disability at discharge (Figure 2; Figure S1). The other 3 patients recovered after medical treatment.
Table 1
| Variables | Non-HPS (n=164) | HPS (n=6) | P value |
|---|---|---|---|
| Age (year)# | 56.90±8.65 | 59.67±8.24 | 0.44 |
| Sex (male), n (%) | 115 (70.1) | 5 (83.3) | 0.67 |
| BMI (kg/m2)¶ | 25.1 (23.6–27.3) | 25.8 (24.1–29.4) | 0.40 |
| Smoking, n (%) | 82 (50.3) | 3 (50.0) | >0.99 |
| Hypertension, n (%) | 120 (73.2) | 5 (83.3) | >0.99 |
| Diabetes mellitus, n (%) | 71 (43.3) | 5 (83.3) | 0.09 |
| Hyperlipidemia, n (%) | 82 (50.0) | 4 (66.7) | 0.68 |
| Coronary artery disease, n (%) | 19 (11.6) | 2 (33.3) | 0.16 |
| Qualifying ischemic events, n (%) | >0.99 | ||
| Ischemic stroke | 127 (77.4) | 5 (83.3) | |
| TIA | 37 (22.6) | 1 (16.7) | |
| Time from qualifying event to endovascular treatment (days)¶ | 66.6 (37.6–179.8) | 104.7 (26.6–274.2) | 0.76 |
| Time from qualifying event to CTP (days)¶ | 55.0 (32.0–161.8) | 103.0 (21.8–248.0) | 0.64 |
| Lesion location, n (%) | 0.67 | ||
| ICA | 61 (37.2) | 3 (50.0) | |
| MCA | 103 (62.8) | 3 (50.0) | |
| Systolic blood pressure at admission, mmHg¶ | 139.1±18.1 | 146.0±1.5 | 0.41 |
| Systolic blood pressure after procedure, mmHg¶ | 128.8±15.2 | 128.7±1.4 | 0.99 |
| Degree of stenosis (%)¶ | 80.0 (70.0–90.0) | 82.5 (73.8–90.0) | 0.84 |
| Length of lesion (mm)¶ | 7.0 (4.8–9.0) | 6.3 (5.8–9.3) | 0.87 |
| Degree of residual stenosis (%)¶ | 10.0 (5.0–15.0) | 15.0 (10.0–16.3) | 0.08 |
| Improvement of stenosis (%)¶ | 70.0 (60.0–80.0) | 70.0 (58.75–70.0) | 0.22 |
| CBF <30% volume (mL)¶ | 0 (0, 0) | 0 (0, 1.3) | 0.17 |
| Tmax >4 s volume (mL)¶ | 93.0 (17.0–177.0) | 429.5 (122.3–582.5) | 0.006* |
| Tmax >6 s volume (mL)¶ | 0 (0–31.8) | 200 (25.5–288.3) | 0.003* |
#, mean ± standard deviation; ¶, median (interquartile range). *, the difference between the two groups was statistically significant. HPS, hyperperfusion syndrome; BMI, body mass index; TIA, transient ischemic attack; CTP, computed tomography perfusion; ICA, internal carotid artery; MCA, middle cerebral artery; CBF, cerebral blood flow; Tmax, time-to-maximum of the residue function.
Table 2
| Patients | Age range (years) | Lesion location | Clinical feature | Time from endovascular treatment to symptom onset | Preprocedural peak blood flow velocity* | Postprocedural peak blood flow velocity* | Tmax >4 s volume | Tmax >6 s volume | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | 50–60 | MCA | ICH | 1 h | – | – | 456 mL | 249 mL | Death |
| Patient 2 | 70–80 | ICA | ICH | 3 h | – | – | 611 mL | 243 mL | Death |
| Patient 3 | 60–70 | ICA | Dysphoria | 0 h | 57 cm/s | 176 cm/s | 573 mL | 406 mL | Recovery |
| Patient 4 | 60–70 | MCA | ICH | 5.5 h | – | – | 403 mL | 157 mL | Severe disability |
| Patient 5 | 60–70 | MCA | Delirium | 17.5 h | 60 cm/s | 265 cm/s | 141 mL | 0 mL | Recovery |
| Patient 6 | 50–60 | ICA | Headache | 2 h | 52 cm/s | 124 cm/s | 66 mL | 34 mL | Recovery |
*, examination for the normal vessel of the distal ipsilateral middle cerebral artery with transcranial Doppler sonography. MCA, middle cerebral artery; ICA, internal carotid artery; ICH, intracranial hemorrhage; Tmax, time-to-maximum of the residue function.
Comparison of patients with HPS and non-HPS
There was no significant difference in demographic characteristics between the two groups (Table 1). There was also no significant difference in the qualifying ischemic event, location of the lesion, systolic blood pressure after the procedure, degree of stenosis, or degree of residual stenosis (Table 1).
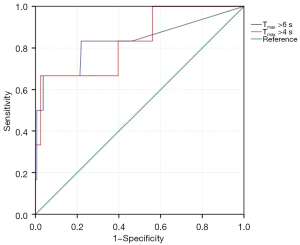
In terms of CTP, the volume of CBF less than 30% showed no difference between the two groups. The HPS group had a significantly higher volume of Tmax >4 s (429.5 vs. 93 mL; P=0.006) and Tmax >6 s (200 vs. 0 mL; P=0.003) compared with the non-HPS group (Table 2). The ROC curve analysis showed an area under the curve (AUC) of 0.832 (95% CI: 0.650 to 1.000; P=0.006) for Tmax >4 s and 0.834 (95% CI: 0.615 to 1.000; P=0.006) for Tmax >6 s (Figure 3). The optimal volume threshold in predicting HPS was 65.5 mL with Tmax >4 s (sensitivity, 100%; specificity, 43.9%).
Discussion
In the current study, we proposed predictors of HPS after stent implantation for ICAS based on CTP. Compared with non-HPS patients, HPS patients had a higher volume of Tmax >4 s and Tmax >6 s, as shown by CTP postprocessed with RAPID software. Tmax >4 s volume may be a predictor of HPS after stent implantation in symptomatic ICAS.
HPS is a fatal complication of revascularization therapy for carotid artery stenosis and has been relatively well-studied in previous reports (6,19). However, few studies have reported the incidence of HPS following stent implantation for ICAS. According to Xu et al. (8), a total of 3.37% of patients with ICAS experienced HPS. The rate of HPS in anterior circulation was 5.1%, which was higher than that of this study (3.53%). The underlying reason for this may be that patients in our study had a longer time interval from the qualifying event to endovascular treatment than those in Xu’s study. An interval of less than 3 weeks was reported as one of the risk factors for HPS after intracranial or carotid arterial stenting (8,20). In our study, the time from the event to intervention in the HPS group was numerically longer than that in the non-HPS, but the difference was not statistically significant. Furthermore, we controlled this risk factor; hence, our study showed a lower incidence of HPS. ICH is a serious presentation of HPS (21). The incidence of ICH in our study was 1.8%, which is similar to the 2.6% of nontechnical-related ICH identified in previous studies on intracranial stenting (19). Despite a low incidence of ICH, most of these patients had a poor prognosis. Three patients experienced ICH in this study, of which 2 died and 1 had a severe disability.
A previous study showed that poor collateral circulation was significantly associated with HPS after endovascular treatment for ICAS (8). CTP is widely used in evaluating cerebral perfusion and hemodynamics in ischemic cerebral diseases, which has been demonstrated to be a valuable predictor of HPS after carotid stenting (10,22-24) In the present study, CTP data were postprocessed using RAPID software, which is a quantitative automated system to assess cerebral hypoperfusion. Tmax >4 s and Tmax>6 s were demonstrated to be optimal for the identification of critically hypoperfused tissue (18). Tmax >4 s, Tmax >6 s, and CBF <30% were used to evaluate the hypoperfusion volume in acute large artery occlusion (14,15). However, CBF less than 30% was 0 mL in 95.3% patients in our study, and it was an unsuitable parameter to reflect the cerebral perfusion injury in the setting of symptomatic ICAS. Therefore, in this study, Tmax >4 s and Tmax >6 s were selected to assess cerebral hypoperfusion before the procedure and explore the optimal threshold to predict HPS after the procedure. The ROC curve analysis showed an AUC of 0.832 (95% CI: 0.650 to 1.000; P=0.006) for Tmax >4 s and 0.834 (95% CI: 0.615 to 1.000; P=0.006) for Tmax >6 s. Considering the low morbidity but high mortality of HPS, the maximizing sensitivity was selected, and the optimal threshold for predicting HPS was 65.5 mL with Tmax >4 s (sensitivity of 100%; specificity of 43.9%). For high-risk patients, staged treatment consisting of primary balloon dilation followed by stent implantation may reduce HPS. Aggressive periprocedural management needs to be implemented for such high-risk patients.
As we know, the recognized pathogenesis of HPS is impaired cerebral autoregulation due to long-term brain tissue hypoperfusion (6). Exorbitant perfusion pressure after relieving stenosis abolishes normal myogenic autoregulation. Oxygen-derived free radicals produced during surgery damage cerebrovascular endothelial function and the blood-brain barrier, resulting in the development of HPS (6,25). It is suggested that brain tissue with over-hypoperfusion is more likely to exhibit impaired cerebral autoregulation, causing HPS when perfusion pressure is sharply restored (26). In this study, the volumes of Tmax >4 s (429.5 vs. 93 mL; P=0.006) and Tmax >6 s (200 vs. 0 mL; P=0.003) in the HPS group were significantly higher than those in the non-HPS group. Furthermore, the volumes of Tmax >4 s of the 3 patients with ICH in our study were far more than 65.5 mL. These findings may indicate a more serious decompensated cerebral autoregulation, resulting in more serious reperfusion injury and cerebral edema once perfusion is restored, which means a worse prognosis (27).
There were some limitations in this study. First, this study was a single-center study, which may have caused selection bias. Second, the hemodynamic parameters of CTP may vary according to different machines, contrast agent type, and injection rate of the agent (28), which may result in unavoidable bias in a retrospective study to influence the veracity of outcomes. Third, a multivariate regression analysis failed to be performed due to the small number of events. Forth, since this was a retrospective analysis, the blood pressure and cardiac function before CTP were not recorded, which may have affected the perfusion data.
Conclusions
HPS is an uncommon complication after stent implantation and has a poor prognosis. Tmax >4 s volume may be a predictor of HPS after stent implantation in symptomatic ICAS, and the optimal volume threshold for maximizing sensitivity is 65.5 mL. Further prospective studies should be conducted to confirm our conclusions.
Acknowledgments
We thank all the patients and healthcare providers who participated in this study.
Funding: This work was supported by the National Natural Science Foundation of China (No. 82171894 to N Ma) and the National Natural Science Foundation of China (No. 81825012 and 81730048 to X Lou).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-682/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-682/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the institutional Ethics Committee of Beijing Tiantan Hospital (No. KY2013-013-01), and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Banerjee C, Chimowitz MI. Stroke Caused by Atherosclerosis of the Major Intracranial Arteries. Circ Res 2017;120:502-13. [Crossref] [PubMed]
- Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med 2011;365:993-1003. [Crossref] [PubMed]
- Alexander MJ, Zauner A, Chaloupka JC, Baxter B, Callison RC, Gupta R, Song SS, Yu W. WEAVE Trial: Final Results in 152 On-Label Patients. Stroke 2019;50:889-94. [Crossref] [PubMed]
- Alexander MJ, Zauner A, Gupta R, Alshekhlee A, Fraser JF, Toth G, Given C, Mackenzie L, Kott B, Hassan AE, Shownkeen H, Baxter BW, Callison RC, Yu W. The WOVEN trial: Wingspan One-year Vascular Events and Neurologic Outcomes. J Neurointerv Surg 2021;13:307-10. [Crossref] [PubMed]
- Meyers PM, Phatouros CC, Higashida RT. Hyperperfusion syndrome after intracranial angioplasty and stent placement. Stroke 2006;37:2210-1. [Crossref] [PubMed]
- van Mook WN, Rennenberg RJ, Schurink GW, van Oostenbrugge RJ, Mess WH, Hofman PA, de Leeuw PW. Cerebral hyperperfusion syndrome. Lancet Neurol 2005;4:877-88. [Crossref] [PubMed]
- Terada T, Tsuura M, Matsumoto H, Masuo O, Tsumoto T, Yamaga H, Ohura Y, Itakura T. Hemorrhagic complications after endovascular therapy for atherosclerotic intracranial arterial stenoses. Neurosurgery 2006;59:310-8; discussion 310-8. [Crossref] [PubMed]
- Xu S, Wu P, Shi H, Ji Z, Dai J. Hyperperfusion Syndrome After Stenting for Intracranial Artery Stenosis. Cell Biochem Biophys 2015;71:1537-42. [Crossref] [PubMed]
- Kaku Y, Yoshimura S, Kokuzawa J. Factors predictive of cerebral hyperperfusion after carotid angioplasty and stent placement. AJNR Am J Neuroradiol 2004;25:1403-8. [PubMed]
- Zhang L, Dai D, Li Z, Duan G, Zhang YW, Yang P, Huang Q, Xu Y, Hong B, Liu J. Risk factors for hyperperfusion-induced intracranial hemorrhage after carotid artery stenting in patients with symptomatic severe carotid stenosis evaluation. J Neurointerv Surg 2019;11:474-8. [Crossref] [PubMed]
- Jiang L, Ai Z, Geng W, Chen H, Zhao B, Su H, Yin X, Chen YC. Predictive value of perfusion weighted imaging for early new lesions after stroke patients receive endovascular treatment. Quant Imaging Med Surg 2021;11:3643-54. [Crossref] [PubMed]
- Kremenova K, Holesta M, Peisker T, Girsa D, Weichet J, Lukavsky J, Malikova H. Is limited-coverage CT perfusion helpful in treatment decision-making in patients with acute ischemic stroke? Quant Imaging Med Surg 2020;10:1908-16. [Crossref] [PubMed]
- Yoshie T, Ueda T, Takada T, Nogoshi S, Fukano T, Hasegawa Y. Prediction of cerebral hyperperfusion syndrome after carotid artery stenting by CT perfusion imaging with acetazolamide challenge. Neuroradiology 2016;58:253-9. [Crossref] [PubMed]
- Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 H after Stroke with a Mismatch between Deficit and Infarct. N Engl J Med 2018;378:11-21. [Crossref] [PubMed]
- Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for Stroke at 6 to 16 H with Selection by Perfusion Imaging. N Engl J Med 2018;378:708-18. [Crossref] [PubMed]
- Goldman-Yassen AE, Straka M, Uhouse M, Dehkharghani S. Normative distribution of posterior circulation tissue time-to-maximum: Effects of anatomic variation, tracer kinetics, and implications for patient selection in posterior circulation ischemic stroke. J Cereb Blood Flow Metab 2021;41:1912-23. [Crossref] [PubMed]
- Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, Frankel MR, Levine SR, Chaturvedi S, Kasner SE, Benesch CG, Sila CA, Jovin TG, Romano JG. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med 2005;352:1305-16. [Crossref] [PubMed]
- Olivot JM, Mlynash M, Thijs VN, Kemp S, Lansberg MG, Wechsler L, Bammer R, Marks MP, Albers GW. Optimal Tmax threshold for predicting penumbral tissue in acute stroke. Stroke 2009;40:469-75. [Crossref] [PubMed]
- Lin YH, Liu HM. Update on cerebral hyperperfusion syndrome. J Neurointerv Surg 2020;12:788-93. [Crossref] [PubMed]
- Abou-Chebl A, Reginelli J, Bajzer CT, Yadav JS. Intensive treatment of hypertension decreases the risk of hyperperfusion and intracerebral hemorrhage following carotid artery stenting. Catheter Cardiovasc Interv 2007;69:690-6. [Crossref] [PubMed]
- Moulakakis KG, Mylonas SN, Sfyroeras GS, Andrikopoulos V. Hyperperfusion syndrome after carotid revascularization. J Vasc Surg 2009;49:1060-8. [Crossref] [PubMed]
- Tseng YC, Hsu HL, Lee TH, Hsieh IC, Chen CJ. Prediction of cerebral hyperperfusion syndrome after carotid stenting: a cerebral perfusion computed tomography study. J Comput Assist Tomogr 2009;33:540-5. [Crossref] [PubMed]
- Yassi N, Parsons MW, Christensen S, Sharma G, Bivard A, Donnan GA, Levi CR, Desmond PM, Davis SM, Campbell BC. Prediction of poststroke hemorrhagic transformation using computed tomography perfusion. Stroke 2013;44:3039-43. [Crossref] [PubMed]
- Campbell BC, Yassi N, Ma H, Sharma G, Salinas S, Churilov L, Meretoja A, Parsons MW, Desmond PM, Lansberg MG, Donnan GA, Davis SM. Imaging selection in ischemic stroke: feasibility of automated CT-perfusion analysis. Int J Stroke 2015;10:51-4. [Crossref] [PubMed]
- Ivens S, Gabriel S, Greenberg G, Friedman A, Shelef I. Blood-brain barrier breakdown as a novel mechanism underlying cerebral hyperperfusion syndrome. J Neurol 2010;257:615-20. [Crossref] [PubMed]
- Waltz AG. Effect of blood pressure on blood flow in ischemic and in nonischemic cerebral cortex. The phenomena of autoregulation and luxury perfusion. Neurology 1968;18:613-21. [Crossref] [PubMed]
- González García A, Moniche F, Escudero-Martínez I, Mancha F, Tomasello A, Ribó M, et al. Clinical Predictors of Hyperperfusion Syndrome Following Carotid Stenting: Results From a National Prospective Multicenter Study. JACC Cardiovasc Interv 2019;12:873-82. [Crossref] [PubMed]
- Silvennoinen HM, Hamberg LM, Valanne L, Hunter GJ. Increasing contrast agent concentration improves enhancement in first-pass CT perfusion. AJNR Am J Neuroradiol 2007;28:1299-303. [Crossref] [PubMed]



