Values of new ultrasonic imaging methods for the diagnosis of apical Takotsubo syndrome
Introduction
Takotsubo syndrome (TTS) was originally reported by Dr. Sato in 1991 (1). It is a multifactorial disease contributed to by several complex and coordinated processes, resulting in a wide spectrum of parallel clinical manifestations, such as chest tightness, chest pain, and shortness of breath (2,3). An electrocardiogram (ECG) usually shows ST-segment elevation, T wave inversion, or an abnormal Q wave, and the concurrent serum cardiac enzyme panel is usually slightly elevated (4). Even when severe discrepancies of coronary artery involvement in the pathogenesis between TTS and acute myocardial infarction (AMI) are identified, their similar clinical symptoms tend to prohibit a clear differentiation of TTS from AMI, and many patients are misdiagnosed (5).
Aside from a nearly normal coronary artery, patients with TTS are characterized by reversible ventricular morphology changes and contractile dysfunction. Multimodality imaging can help capture these changes (6). Templin et al. (3) proposed 4 different morphological patterns of TTS: apical, midventricular, basal, and focal type, with the apical contraction abnormality pattern being the most common type (81.7%).
Echocardiography is considered an indispensable imaging modality for patients with TTS because it is convenient, quick, safe, and noninvasive (7). However, the inherent limitations of the traditional echocardiography derived from 2-dimensional (2D) views preclude its use in fully evaluating cardiac morphological changes and myocardial dysfunction. Real-time 3D echocardiography (RT-3DE) and speckle tracking imaging (STI) are new modalities that have recently been introduced into clinical practice (8). RT-3DE does not assume the chamber geometry, can visually display the cardiac space structure and ventricular wall motion, and can provide direct measurement of the cardiac capacity and function (9,10). STI can be used to quantify all aspects of myocardial strain related to myocardial deformation and contractile function by measuring the displacement between 2 different points in a myocardial segment (11). As a new method, STI can avoid interfering with the traction of the surrounding myocardium and heart movement and also eliminate the dependence of tissue Doppler imaging on the angle of the sound beam (12,13).
As more is learned about TTS, related studies are increasingly presented and the literature updated. However, research concerning the recovery procedure of TTS using new echocardiography techniques is relatively scarce. After the left ventricular ejection fraction (LVEF) returns to normal, questions remain about whether the functional recovery of the myocardium occurs simultaneously. Therefore, this study aimed to investigate the ultrasonic characteristics of TTS during different periods and to assess the value of the clinical application of RT-3DE and STI. We present the following article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-21-1108/rc).
Methods
Study population and design
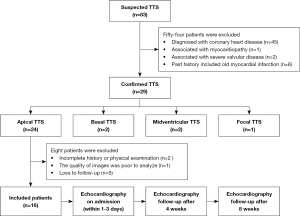
This was a prospective cohort study. Between May 2011 and September 2021, 83 patients with highly suspected TTS were enrolled. These patients were sourced from 3 hospitals: the Second Medical Center of Chinese PLA General Hospital, the First Medical Center of Chinese PLA General Hospital, and The Second Affiliated Hospital of Dalian Medical University. Blood tests, ECG, echocardiography, and invasive coronary angiography were carried out for all patients. RT-3DE and STI were performed on admission for patients diagnosed with apical TTS (within 1–3 days), and these patients were followed up after 4 and 8 weeks. Ultimately, 16 patients were included in this study (Figure 1). They were in accordance with the relevant diagnostic criteria proposed by clinical experts (14,15). Patients were excluded if they had (I) evidence of pheochromocytoma or myocarditis, (II) acute or chronic infectious diseases or other inflammatory conditions, (III) any concurrent physical illness that might be a potential confounder in this study (e.g., concurrent severe valvular disease, hypertrophic cardiomyopathy, non-compaction cardiomyopathy, and so on), (IV) incomplete medical information, or if they were (V) lost to follow-up. During the same period, 20 healthy people, matched for age and sex, were included as normal controls. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Ethics Committee of Chinese PLA General Hospital (No. S2021-276-01). Written informed consent was obtained from all patients.
Conventional echocardiography
Echocardiography was performed using commercially available ultrasound equipment (M3S and 3V with a 1.5–3.4 MHz transducer, GE Vivid 7; M5-1 and X5-1 with a 2.5–4.0 MHz transducer, Philips EPIC 7 and Philips EPIC 7C). An EchoPAC Clinical Workstation (GE Healthcare) equipped with 4D LV Volume Tom Tec and 2D strain analysis software was used for image processing.
All patients were placed in the left lateral decubitus position and echocardiography was acquired with a simultaneous ECG signal. Patients were instructed to breathe calmly. Conventional echocardiography was performed first using all standard views. All measurements were made in accordance with the current guidelines of the European Society of Echocardiography (16). Routine parameters, for example, left atrial diameter (LAD), LV end-diastolic diameter (LVEDD), interventricular septal end-diastolic thickness (IVST), and LV posterior wall thickness (LVPWT), were measured. LVEF was calculated using the biplane method of disks (the modified Simpson’s rule) for 2D echocardiography. Mitral inflow peak E-wave velocity and peak A-wave velocity were measured using pulsed Doppler imaging. Mitral annular peak E’-wave velocity and peak A’-wave velocity were measured using tissue Doppler imaging. Then, the E/A, E’/A’, and E/E’ ratios were acquired by calculation.
RT-3DE examination
LV 3-plane views and full-volume views were acquired during end-expiratory breath-hold using a matrix-array transducer. The LV long axis, the apical 4-chamber, and the apical 2-chamber views of (LV) were acquired simultaneously during 1 cardiac cycle in the 3-plane mode. This allowed us to observe ventricular morphology and wall motions more conveniently and accurately. A clear apical 4-chamber image was obtained, the patients were asked to hold their breath at the end of a breath, and “full-volume” views were acquired. The best full-volume views were collected for 4 consecutive cardiac cycles. These datasets were stored and transferred to a computer for offline analysis. Morphology analysis was performed using a crop box, and full-volume views could be sectioned in any desired direction and angulation. Finally, the analysis software (4D-LV Volume Tom Tec) was used to analyze all the collected full-volume images. The tracks of the endocardial borders in end-systole and end-diastole were detected by the software. Finally, the system provided the LVEDV, LVESV, LVSV and LVEF.
STI examination
Two-dimensional high-frame-rate (60–80 frames/s) images were collected from the LV long-axis, the apical 4-chamber, and the apical 2-chamber views of the LV during an end-expiratory breath hold. Three consecutive cardiac cycles were acquired at each view and saved in a cine-loop format for offline analysis with 2D strain software. An LV segmental model specified by the American and European Society of Echocardiography was used (16). LV endocardial borders were manually traced in the end-systolic phase for strain analysis, and the regions of interest were adjusted to fit the whole myocardium. The automatic algorithm provided the longitudinal peak systolic strain (LPSS) for each LV segment. LPSS was expressed as a negative value. A less negative LPSS value meant a lower systolic function. The average LPSS values of the global and regional (basal, middle, and apical) segments were calculated. Longitudinal strain results for the individual were visualized in a color-coded format polar map (red-pink-blue) and combined intuitively in a bull’s-eye plot. The inner ring represented the apex of the LV, the middle ring the middle segments, and the outer ring the base. Bright red meant normal strain values (<−16%), light red meant declined values (−16% to −11%), light pink (−10% to −6%) and pale pink (−5% to 0) meant heavily reduced values, and blue meant positive values that suggested paradoxical systolic expansion (17).
Statistical analysis
Data analysis was performed using SPSS 22.0 (IBM Corp, Armonk, NY, USA) software. PASS 15.0 (NCSS LLC, Kaysville, UT, USA) was used to calculate the sample, and the current study met the needs of the minimum sample size. Results are presented as mean ± standard deviation (mean ± SD). A normality test was used to compare continuous variables, and a 1-way analysis of variance was used to compare the echocardiographic values of patients with TTS and normal controls. Continuous variables from different groups were compared using the Fisher least significant difference test. All P values were 2-sided, and values less than 0.05 were considered statistically significant.
Results
Clinical characteristics and general parameters
Ultimately, 16 apical TTS patients were included in this study. They were aged 41–90 years (the mean age was 62.75±12.26 years), 94% were women, and 6% were men. The majority of the women (87%) were postmenopausal. All patients had invasive coronary angiography and an absence of obstructive coronary artery disease or angiographic evidence of acute plaque rupture (Figure 2). The baseline clinical information is shown in Table 1. Emotional stressors were responsible for TTS in 81% of cases, and physical stressors were responsible for TTS in 19% of cases. All patients had chest tightness (100%), 69% experienced angina, 56% had dyspnea, and they all had increased troponin I (100%) and creatine kinase isoenzyme (100%). The rate of ECG abnormalities with ST-elevation was found to be 75%, and, to a lesser extent, T wave inversion (56%) and others (31%). LVEF-2DE was significantly lower in patients with TTS than in normal controls (37.42%±5.74% vs. 65.36%±3.31%; P<0.001), whereas no significant differences in LAD, LVEDD, IVST, or LVPWT were found (36.02±2.37 vs. 35.29±1.64 mm, P=0.283; 46.18±3.56 vs. 44.34±2.03 mm, P=0.059; 9.35±0.33 vs. 9.54±0.28 mm, P=0.070; 9.17±0.35 vs. 9.31±0.25 mm, P=0.171; Table 2).
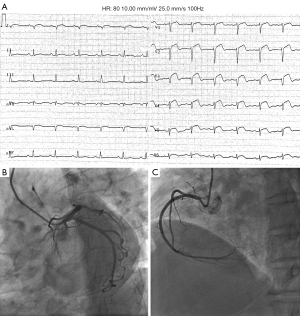
Table 1
| Parameters | Normal controls (n=20) | TTS (n=16) |
|---|---|---|
| Female, n [%] | 19 [95] | 15 [94] |
| Postmenopausal | 17 [89] | 13 [87] |
| Premenopausal | 2 [11] | 2 [13] |
| Presenting symptom, n [%] | ||
| Chest tightness | 0 | 16 [100] |
| Angina | 0 | 11 [69] |
| Dyspnea | 0 | 9 [56] |
| Serum myocardial enzyme increase, n [%] | ||
| Troponin I | 0 | 16 [100] |
| Creatine kinase isoenzyme | 0 | 16 [100] |
| ECG upon presentation, n [%] | ||
| ST-elevation | 0 | 12 [75] |
| T wave inversion | 0 | 9 [56] |
| Other | 0 | 5 [31] |
| Triggering factor, n [%] | ||
| Physical stress (e.g., bodily injury, critical illness, surgery) | 0 | 3 [19] |
| Emotional stress (e.g., quarrel, bereavement, domestic calamity) | 0 | 13 [81] |
TTS, Takotsubo syndrome; ECG, electrocardiogram.
Table 2
| Parameters | Normal controls (n=20) | TTS (n=16) | t/P |
|---|---|---|---|
| Age (years) | 62.75±12.26 | 60.34±11.79 | 0.596/0.555 |
| LAD (mm) | 35.29±1.64 | 36.02±2.37 | 1.091/0.283 |
| LVEDD (mm) | 44.34±2.03 | 46.18±3.56 | 1.952/0.059 |
| IVST (mm) | 9.54±0.28 | 9.35±0.33 | 1.869/0.070 |
| LVPWT (mm) | 9.31±0.25 | 9.17±0.35 | 1.399/0.171 |
| LVEF-2DE (%) | 65.36±3.31 | 37.42±5.74 | 18.328/<0.001 |
TTS, Takotsubo syndrome; SD, standard deviation; LAD, left atrial diameter; LVEDD, left ventricular end-diastolic diameter; IVST, interventricular septal end-diastolic thickness; LVPWT, left ventricular posterior wall thickness; LVEF-2DE, left ventricular ejection fraction by 2-dimensional echocardiography.
LV systolic and diastolic function parameters
Tables 3,4 list the differences in volumetric parameters and ejection fraction between patients with TTS and normal controls. LVEDV, LVESV, LVSV, and LVEF calculated from RT-3DE datasets were 84.23±10.67, 55.94±8.51, 28.31±8.06 mL, and 33.59%±4.12% in patients with TTS on admission. These values were significantly different from those of normal controls (P=0.005, P<0.001, P<0.001, and P<0.001, respectively). During the follow-up period, these parameters gradually recovered. After 8 weeks, a significant recovery was found in LVEDV 75.79±6.86 mL, LVESV 28.05±4.33 mL, LVSV 47.81±3.57 mL, and LVEF 63.02%±3.92%, and there were no significant differences compared with normal controls (P=0.907, P=0.235, P=0.162, and P=0.052, respectively). Mitral inflow peak E-wave velocity and mitral annular peak E’-wave velocity significantly decreased (both P values <0.001), while mitral inflow peak A-wave velocity and mitral annular peak A’-wave significantly increased (both P values <0.05) on admission and after 4 and 8 weeks, compared with normal controls. There was no obvious variation of these values in patients with TTS during different periods. In normal controls, the E/A ratio was >1 while the E’/A’ ratio was >1, but in patients with TTS the E/A ratio was <1 while the E’/A’ ratio was >1 during different periods. Compared with normal controls, the E/E’ ratio was markedly elevated on admission (P=0.001), while this value showed no significant differences after 8 weeks (P=0.972).
Table 3
| Parameters | Normal controls (n=20) | TTS (n=16) | ||
|---|---|---|---|---|
| On admission | After 4 weeks | After 8 weeks | ||
| LVEDV (mL) | 76.03±5.38 | 84.23±10.67 | 78.92±7.04 | 75.79±6.86 |
| LVESV (mL) | 26.47±3.52 | 55.94±8.51 | 33.04±5.68 | 28.05±4.33 |
| LVSV (mL) | 49.56±3.71 | 28.31±8.06 | 45.86±5.91 | 47.81±3.57 |
| LVEF (%) | 65.18±2.49 | 33.59±4.12 | 58.09±3.26 | 63.02±3.92 |
| E (m/s) | 0.83±0.16 | 0.58±0.09 | 0.51±0.11 | 0.55±0.11 |
| A (m/s) | 0.67±0.11 | 0.81±0.09 | 0.79±0.10 | 0.78±0.09 |
| E/A ratio | 1.23±0.09 | 0.71±0.06 | 0.64±0.05 | 0.70±0.08 |
| E’ (cm/s) | 8.57±2.13 | 4.93±1.34 | 5.05±1.18 | 5.67±1.41 |
| A’ (cm/s) | 6.11±2.45 | 8.16±1.70 | 8.03±1.49 | 7.95±1.33 |
| E’/A’ ratio | 1.40±0.12 | 0.61±0.07 | 0.63±0.06 | 0.71±0.08 |
| E/E’ ratio | 9.68±1.47 | 11.70±2.01 | 10.1±2.15 | 9.70±1.96 |
LVEDV, LVESV, LVSV, and LVEF were measured by real-time 3-dimensional echocardiography. TTS, Takotsubo syndrome; SD, standard deviation; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; LVSV, left ventricular stroke volume; LVEF, left ventricular ejection fraction; E, mitral inflow peak E-wave velocity; A, mitral inflow peak A-wave velocity; E’, mitral annular peak E’-wave velocity; A’, mitral annular peak A’-wave velocity.
Table 4
| Parameters | t1/P1 | t2/P2 | t3/P3 | t4/P4 | t5/P5 | t6/P6 |
|---|---|---|---|---|---|---|
| LVEDV (mL) | 3.000/0.005 | 1.397/0.171 | 0.118/0.907 | 1.662/0.107 | 2.661/0.012 | 1.273/0.213 |
| LVESV (mL) | 14.092/<0.001 | 4.249/<0.001 | 1.208/0.235 | 8.953/<0.001 | 11.684/<0.001 | 2.795/0.009 |
| LVSV (mL) | 10.507/<0.001 | 2.295/0.028 | 1.429/0.162 | 7.023/<0.001 | 8.849/<0.001 | 1.129/0.268 |
| LVEF (%) | 28.458/<0.001 | 7.403/<0.001 | 2.012/0.052 | 18.653/<0.001 | 21.700/<0.001 | 3.868/0.001 |
| E (m/s) | 5.574/<0.001 | 6.807/<0.001 | 5.956/<0.001 | 1.97/0.058 | 0.844/0.405 | 1.028/0.312 |
| A (m/s) | 4.106/<0.001 | 3.384/0.002 | 3.335/0.003 | 0.594/0.557 | 0.942/0.353 | 0.297/0.768 |
| E/A ratio | 19.826/<0.001 | 23.444/<0.001 | 18.431/<0.001 | 3.585/0.001 | 0.400/0.692 | 2.543/0.016 |
| E’ (cm/s) | 5.949/<0.001 | 5.913/<0.001 | 4.680/<0.001 | 0.268/0.790 | 1.521/0.139 | 1.348/0.187 |
| A’ (cm/s) | 2.841/<0.001 | 2.749/<0.001 | 2.697/<0.001 | 0.230/0.820 | 0.389/0.700 | 0.160/0.874 |
| E’/A’ ratio | 23.311/<0.001 | 23.387/<0.001 | 19.73/<0.001 | 0.867/0.392 | 3.762/0.001 | 3.200/0.003 |
| E/E’ ratio | 3.483/0.001 | 0.695/0.490 | 0.035/0.972 | 2.174/0.038 | 2.849/0.008 | 0.549/0.586 |
The t1/P1, t2/P2, and t3/P3 were patients with TTS on admission and after 4 and 8 weeks vs. normal controls. The t4/P4 and t5/P5 were patients with TTS after 4 and 8 weeks vs. on admission. The t6/P6 was TTS patients after 8 weeks vs. after 4 weeks. LVESV, LVSV, and LVEF were measured by real-time 3-dimensional echocardiography. TTS, Takotsubo syndrome; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; LVSV, left ventricular stroke volume; LVEF, left ventricular ejection fraction; E, mitral inflow peak E-wave velocity; A, mitral inflow peak A-wave velocity; E’, mitral annular peak E’-wave velocity; A’, mitral annular peak A’-wave velocity.
LV morphology, wall motion, and longitudinal strain
On admission, the LV morphology resembled an “octopus pot” with a round bottom (apical segments) and a narrow neck (basal segments). LV akinetic myocardial segments were beyond the territory of a single coronary artery distribution. In the bull’s-eye plot, blue, which indicated paradoxical systolic expansion, was visible in the apex, and even spread to the part of middle segments (Figure 3). LPSS decreased significantly in all segments, even in the basal segments that were hyperkinetic or normal in conventional echocardiography compared with normal controls (−14.95%±3.01% vs. −21.51%±2.85%; P<0.001). After 4 weeks, significant improvements were noted, but the apex was still slightly abnormal and was light red or pink. After 8 weeks, all the views showed a normal LV shape. There were no significant differences in LPSS in the basal segments (−20.75%±2.91% vs. −21.51%±2.85%; P=0.055), whereas LPSS in the apical and middle segments were still significantly lower than that of the normal controls (−18.54%±4.69% vs. −24.29%±3.46%, P<0.001; −19.38%±2.88% vs. −22.36%±3.23%, P<0.001; Tables 5,6).
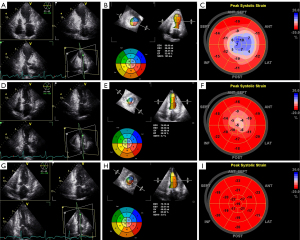
Table 5
| LPSS (%) | Normal controls | TTS | ||
|---|---|---|---|---|
| On admission | After 4 weeks | After 8 weeks | ||
| Apical segments | –24.29±3.46 | –4.43±1.72 | –11.71±3.43 | –18.54±4.69 |
| Middle segments | –22.36±3.23 | –10.68±2.79 | –14.02±3.09 | –19.38±2.88 |
| Basal segments | –21.51±2.85 | –14.95±3.01 | –18.53±2.78 | –20.75±2.91 |
| Global segments | –22.72±3.27 | –9.97±4.32 | –14.77±4.06 | –19.54±3.56 |
TTS, Takotsubo syndrome; SD, standard deviation; LPSS, longitudinal peak systolic strain.
Table 6
| Parameters | t1/P1 | t2/P2 | t3/P3 | t4/P4 | t5/P5 | t6/P6 |
|---|---|---|---|---|---|---|
| Apical segments | 51.373/<0.001 | 26.655/<0.001 | 10.362/<0.001 | 18.589/<0.001 | 27.675/<0.001 | 1.517/<0.001 |
| Middle segments | 27.552/<0.001 | 18.916/<0.001 | 6.948/<0.001 | 7.861/<0.001 | 21.258/<0.001 | 12.432/<0.001 |
| Basal segments | 16.395/<0.001 | 7.694/<0.001 | 1.929/0.055 | 8.585/<0.001 | 13.574/<0.001 | 5.380/<0.001 |
| Global segments | 42.748/<0.001 | 27.609/<0.001 | 11.824/<0.001 | 13.740/<0.001 | 29.013/<0.001 | 14.991/<0.001 |
The t1/P1, t2/P2, and t3/P3 were patients with TTS on admission and after 4 and 8 weeks vs. normal controls. The t4/P4 and t5/P5 were patients with TTS after 4 and 8 weeks vs. on admission. The t6/P6 patients with TTS after 8 weeks vs. after 4 weeks. TTS, Takotsubo syndrome; LPSS, longitudinal peak systolic strain.
Discussion
TTS, once considered a rare disease, has now gained more recognition. Reports indicate that the proportion of TTS with a suspected AMI has increased to approximately 0.7–2.5%, other studies have even reported higher rates of up to 7.5–12.0%, with a higher incidence in women, especially postmenopausal women (3,5,18-20). In this study, we found that 94% of patients were women, 87% of whom were postmenopausal. One possible explanation for this is that the levels of estrogen, a protective hormone, might plummet after menopause, leading to endothelial dysfunction, sympathetic drive, and an increase in oxidative stress markers and neuropeptide Y, all of which render postmenopausal women more vulnerable to coronary vasoreactivity dysfunction (21,22). Multiple other factors could also contribute to TTS, including emotional or physical stressors. In this study, emotional stress (e.g., quarrel, bereavement, domestic calamity) seemed more common than physical stressors (81% vs. 19%). This is in line with findings suggesting women are more affected than men by negative thoughts, depression, anxiety, sadness, and sleep disorders, all of which are common among postmenopausal women (23,24).
In this study, the whole LV morphology in patients with TTS on admission showed a typical apical ballooning pattern, also known as “octopus pot”, with a widespread apex and/or middle myocardial motion hypokinesis (a circumferential pattern) beyond the territory of a single coronary artery distribution. However, there were no significant differences in LVEDD, IVST, or LVPWT compared with normal controls (P>0.05). The reason may be that measurement of these LV parameters was acquired in the parasternal long-axis view, which was obtained perpendicular to the LV long axis and measured at the level of the mitral valve leaflet tips. These parameters are representative only in normally shaped ventricles, but for patients with apical TTS, LV apical changes are a prominent feature (16). Thus, we should choose more appropriate parameters and images to achieve the best performance. RT-3DE can reveal the cardiac stereoscopic structure without reliance on geometric assumptions and is more objective and accurate for assessing LV shape and function (25). Compared with normal controls, LVEDV and LVESV significantly increased, and LVSV and LVEF severely decreased in RT-3DE.
Fortunately, these structural and functional abnormalities were transient and reversible. We observed some interesting and promising changes during the follow-up period, even after 8 weeks. One was that the LV of patients with TTS returned to the normal vertebral shape without visible wall motion hypokinesis. LVEF measured by RT-3DE also rose to the normal range. The drastic changes observed might be related to the pathophysiology of TTS. The most widely accepted theoretical basis for the pathophysiology is that of direct catecholamine induction, in which myocyte injury is caused by plasma catecholamines and various neuropeptides (e.g., epinephrine, norepinephrine, and dopamine) (5,18,26). β-2-adrenoceptor coupling from Gs to Gi is stimulated, which aggravates the cardiac negative inotropy and contractile dysfunction (27). The specific abnormalities that primarily occur at the apical and/or midventricular segments may depend on the distribution of β-2-adrenergic receptors. The heart has a higher concentration of the β-2-adrenergic receptors in the apical myocardium, with the concentration gradient decreasing from apex to base (4,7). Acute β-adrenergic overload produces myocyte damage through calcium leakage from the ryanodine receptor 2 but spares cardiac stem cells (28). We believe that LVEF recovered rapidly with spares cardiac stem cells undamaged.
Furthermore, in patients with TTS during different periods, the E/A ratio <1 and the E’/A’ ratio >1 suggested that LV diastole function decreased. However, filling patterns in older adults, even in healthy ones, resemble those observed in mild diastolic dysfunction (29). In this study, we showed that the E/E’ ratio was a unique and valuable marker of diastolic function. Elevated E/E’ ratio suggested diastolic dysfunction because of abnormal LV stiffness and filling in TTS patients on admission (30). However, with the improvement of patients with TTS, the E/E’ ratio dropped off, which resulted in no significant differences after 8 weeks (P=0.972). Therefore, we believe that E/E ratio correlates with LV end-diastolic pressure and can be used for the follow-up and recovery of patients with TTS patient.
It is worth noting that the longitudinal strain of the base, which was once thought to dictate hypercontractility or normal contractility in general, also decreased significantly in patients with apical TTS on admission (P<0.001). We believe that excessive catecholamines and neuropeptides have a direct toxic effect on cardiomyocytes, including the basal segment. The basal myocardial injury is less severe and often difficult to discern visually. STI represents a new, powerful, and sensitive method for quantifying myocardial mechanical function and myocardial injury (31). The global LV volume function almost normalized, and wall motion abnormalities were no longer visible after 8 weeks, but the longitudinal strain of the apical and middle segments remained distinctly decreased compared with that in normal controls, especially apical segments. This may be related to the myocardial band structure, which showed a spiral ventricular myocardial band comprising the basal loop that extended longitudinally, from the base to the apex, wrapping around the ventricular cavity to the apex, and then forming the descending component of the apical loop (32-34). Owing to the special anatomical structure of the LV and its sympathetic innervation (as mentioned before), the apex was the most damaged region. Despite the survival of cardiac stem cells, other effects of cardiotoxicity, including inflammatory cell infiltration, contraction band necrosis, and fibrosis, have already been confirmed (3). This could be why some parameters did not completely return to normal in the short term of 8 weeks.
Limitations
First, we realize that the relatively small sample size is the primary limitation of this study. Although TTS is not as rare as when its clinical diagnosis was first developed, it remains a rather uncommon disease. Therefore, it is worth studying in more detail. Second, TTS has a significant female predominance, especially in postmenopausal women. Future research should focus more intensely on older females but should be cautious not to overlook the condition in men. Third, the echocardiographic data were obtained from machines manufactured by different vendors (Philips Healthcare and GE Healthcare). The variability among vendors might have introduced greater uncertainty into the findings. However, previous studies confirmed good correlations between the values of longitudinal strain obtained with the Philips and GE echocardiographic systems (17,35). Therefore, this variability may not have significantly affected the results. In addition, despite the considerable advantages of two-dimensional STI, the technology is far from perfect and still depends on the quality of the images. The planar movement, frames, and tracking quality can all produce inaccuracies.
Conclusions
Patients with the apical TTS typically had an “octopus pot” morphology on images accompanied by serious cardiac function insufficiency and widespread motion hypokinesis in the apical and/or middle part of the myocardium, all of which gradually recovered in the follow-up period. The longitudinal strain of the basal segments also decreased significantly at the onset of apical TTS. Another notable result from this study was the longitudinal strain of the apical and middle segments, which did not fully recover within the short duration of 8 weeks, even though LV morphology and wall motion returned to normal. RT-3DE and STI play an important role in visually and quantitatively inspecting these abnormalities, thus contributing to improved diagnosis and follow-up.
Recommendations
We recommend sonographers store LV long-axis, apical 4-chamber, and apical 2-chamber views during routine echocardiography for patients with suspected or confirmed TTS. The values of LV longitudinal strain can easily be acquired using analysis software. If the 3D echocardiography probe is equipped, the full volume data of the heart can be captured for further analysis, including LV shape, LV volume, LVEF, and so on. We believe that implementing these measures will benefit both patients and clinicians.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-21-1108/rc).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-21-1108/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of approved by the Ethics Committee of Chinese PLA General Hospital (No. S2021-276-01). Written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dote K, Sato H, Tateishi H, Uchida T, Ishihara M. J Cardiol 1991;21:203-14. [Myocardial stunning due to simultaneous multivessel coronary spasms: a review of 5 cases]. [PubMed]
- Arcari L, Musumeci MB, Stiermaier T, El-Battrawy I, Moller C, Guerra F, Novo G, Mariano E, Limite LR, Cacciotti L, Semeraro R, Volpe M, Romeo F, Caldarola P, Thiele H, Akin I, Brunetti ND, Eitel I, Santoro F. Incidence, determinants and prognostic relevance of dyspnea at admission in patients with Takotsubo syndrome: results from the international multicenter GEIST registry. Sci Rep 2020;10:13603. [Crossref] [PubMed]
- Templin C, Ghadri JR, Diekmann J, Napp LC, Bataiosu DR, Jaguszewski M, Cammann VL, Sarcon A, Geyer V, Neumann CA, Seifert B, Hellermann J, Schwyzer M, Eisenhardt K, Jenewein J, Franke J, Katus HA, Burgdorf C, Schunkert H, Moeller C, Thiele H, Bauersachs J, Tschope C, Schultheiss HP, Laney CA, Rajan L, Michels G, Pfister R, Ukena C, Bohm M, Erbel R, Cuneo A, Kuck KH, Jacobshagen C, Hasenfuss G, Karakas M, Koenig W, Rottbauer W, Said SM, Braun-Dullaeus RC, Cuculi F, Banning A, Fischer TA, Vasankari T, Airaksinen KE, Fijalkowski M, Rynkiewicz A, Pawlak M, Opolski G, Dworakowski R, MacCarthy P, Kaiser C, Osswald S, Galiuto L, Crea F, Dichtl W, Franz WM, Empen K, Felix SB, Delmas C, Lairez O, Erne P, Bax JJ, Ford I, Ruschitzka F, Prasad A, Luscher TF. Clinical Features and Outcomes of Takotsubo (Stress) Cardiomyopathy. N Engl J Med 2015;373:929-38. [Crossref] [PubMed]
- Moscatelli S, Montecucco F, Carbone F, Valbusa A, Massobrio L, Porto I, Brunelli C, Rosa GM. An Emerging Cardiovascular Disease: Takotsubo Syndrome. Biomed Res Int 2019;2019:6571045. [Crossref] [PubMed]
- Napp LC, Cammann VL, Jaguszewski M, Szawan KA, Wischnewsky M, Gili S, et al. Coexistence and outcome of coronary artery disease in Takotsubo syndrome. Eur Heart J 2020;41:3255-68. [Crossref] [PubMed]
- Citro R, Okura H, Ghadri JR, Izumi C, Meimoun P, Izumo M, Dawson D, Kaji S, Eitel I, Kagiyama N, Kobayashi Y, Templin C, Delgado V, Nakatani S, Popescu BA, Committee ESD. Multimodality imaging in takotsubo syndrome: a joint consensus document of the European Association of Cardiovascular Imaging (EACVI) and the Japanese Society of Echocardiography (JSE). Eur Heart J Cardiovasc Imaging 2020;21:1184-207. [Crossref] [PubMed]
- Izumo M, Akashi YJ. Role of echocardiography for takotsubo cardiomyopathy: clinical and prognostic implications. Cardiovasc Diagn Ther 2018;8:90-100. [Crossref] [PubMed]
- Abd El Rahman M, Jung AM, Zemlin M, Rohrer TR, Schuck R, Oberhoffer FS, Abdul-Khaliq H. Left atrial remodelling among Turner syndrome patients: novel insights from non-invasive 3D echocardiography. Quant Imaging Med Surg 2022;12:2634-48. [Crossref] [PubMed]
- Jiang F, Chen Y, Wu L, Zhang Y, Liu J, Sun X, Li J, Mao M, Yang S. Left heart function evaluation of patients with essential hypertension and paroxysmal atrial fibrillation by two-dimensional speckle tracking imaging combined with real-time three-dimensional ultrasound imaging. J Thorac Dis 2021;13:322-33. [Crossref] [PubMed]
- Mancuso FJN. Real-Time Three-Dimensional Echocardiography and Myocardial Strain: Ready for Use in Clinical Practice. Arq Bras Cardiol 2019;113:946-7. [Crossref] [PubMed]
- Charfeddine S, Abid L, Hammami R, Bahloul A, Triki F, Kammoun S. Left ventricular myocardial function in hemodialysis patients: the effects of preload decrease in conventional, Doppler and speckle tracking echocardiography parameters. Pan Afr Med J 2021;38:45. [Crossref] [PubMed]
- Wang X, Hong J, Zhang T, Xu D. Changes in left ventricular and atrial mechanics and function after dialysis in patients with end-stage renal disease. Quant Imaging Med Surg 2021;11:1899-908. [Crossref] [PubMed]
- Suzuki K, Kato T, Koyama S, Shinohara T, Inukai S, Sato J, Yamamoto H, Omori D, Yoshida S, Takeda S, Nishikawa H, Ohashi N, Sakurai H, Saitoh S. Influence of Percutaneous Occlusion of Atrial Septal Defect on Left Atrial Function Evaluated Using 2D Speckle Tracking Echocardiography. Int Heart J 2020;61:83-8. [Crossref] [PubMed]
- Kawai S, Kitabatake A, Tomoike H, Takotsubo Cardiomyopathy G. Guidelines for diagnosis of takotsubo (ampulla) cardiomyopathy. Circ J 2007;71:990-2. [Crossref] [PubMed]
- Ghadri JR, Wittstein IS, Prasad A, Sharkey S, Dote K, Akashi YJ, Cammann VL, Crea F, Galiuto L, Desmet W, Yoshida T, Manfredini R, Eitel I, Kosuge M, Nef HM, Deshmukh A, Lerman A, Bossone E, Citro R, Ueyama T, Corrado D, Kurisu S, Ruschitzka F, Winchester D, Lyon AR, Omerovic E, Bax JJ, Meimoun P, Tarantini G, Rihal C. S YH, Migliore F, Horowitz JD, Shimokawa H, Luscher TF, Templin C. International Expert Consensus Document on Takotsubo Syndrome (Part I): Clinical Characteristics, Diagnostic Criteria, and Pathophysiology. Eur Heart J 2018;39:2032-46. [Crossref] [PubMed]
- Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233-70. [Crossref] [PubMed]
- Liu D, Hu K, Nordbeck P, Ertl G, Stork S, Weidemann F. Longitudinal strain bull's eye plot patterns in patients with cardiomyopathy and concentric left ventricular hypertrophy. Eur J Med Res 2016;21:21. [Crossref] [PubMed]
- Pilgrim TM, Wyss TR. Takotsubo cardiomyopathy or transient left ventricular apical ballooning syndrome: A systematic review. Int J Cardiol 2008;124:283-92. [Crossref] [PubMed]
- Medina de Chazal H, Del Buono MG, Keyser-Marcus L, Ma L, Moeller FG, Berrocal D, Abbate A. Stress Cardiomyopathy Diagnosis and Treatment: JACC State-of-the-Art Review. J Am Coll Cardiol 2018;72:1955-71. [Crossref] [PubMed]
- Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimsky P, Group ESCSD. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018;39:119-77. [Crossref] [PubMed]
- Vitale C, Mendelsohn ME, Rosano GM. Gender differences in the cardiovascular effect of sex hormones. Nat Rev Cardiol 2009;6:532-42. [Crossref] [PubMed]
- Sánchez-Rodríguez MA, Castrejon-Delgado L, Zacarias-Flores M, Arronte-Rosales A, Mendoza-Nunez VM. Quality of life among post-menopausal women due to oxidative stress boosted by dysthymia and anxiety. BMC Womens Health 2017;17:1. [Crossref] [PubMed]
- Albert K, Ledet T, Taylor W, Newhouse P. Estradiol administration differentially affects the response to experimental psychosocial stress in post-menopausal women with or without a history of major depression. J Affect Disord 2020;261:204-10. [Crossref] [PubMed]
- Soares CN, Zitek B. Reproductive hormone sensitivity and risk for depression across the female life cycle: a continuum of vulnerability? J Psychiatry Neurosci 2008;33:331-43. [PubMed]
- Italiano G, Fusini L, Mantegazza V, Tamborini G, Muratori M, Ghulam Ali S, Penso M, Garlasche A, Gripari P, Pepi M. Novelties in 3D Transthoracic Echocardiography. J Clin Med 2021;10. [PubMed]
- Wittstein IS, Thiemann DR, Lima JA, Baughman KL, Schulman SP, Gerstenblith G, Wu KC, Rade JJ, Bivalacqua TJ, Champion HC. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med 2005;352:539-48. [Crossref] [PubMed]
- Khalid N, Ahmad SA, Shlofmitz E, Chhabra L. Pathophysiology of Takotsubo Syndrome. Treasure Island, FL, USA: StatPearls, 2021.
- Ellison GM, Torella D, Karakikes I, Purushothaman S, Curcio A, Gasparri C, Indolfi C, Cable NT, Goldspink DF, Nadal-Ginard B. Acute beta-adrenergic overload produces myocyte damage through calcium leakage from the ryanodine receptor 2 but spares cardiac stem cells. J Biol Chem 2007;282:11397-409. [Crossref] [PubMed]
- Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Alexandru Popescu B, Waggoner AD, Houston T, Oslo N, Phoenix A, Nashville T, Hamilton OC, Uppsala S. Ghent, Liege B, Cleveland O, Novara I, Rochester M, Bucharest R, St. Louis M. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016;17:1321-60. [Crossref] [PubMed]
- Medeiros K, O'Connor MJ, Baicu CF, Fitzgibbons TP, Shaw P, Tighe DA, Zile MR, Aurigemma GP. Systolic and diastolic mechanics in stress cardiomyopathy. Circulation 2014;129:1659-67. [Crossref] [PubMed]
- Ahmed M, Sardana M, Rasla S, Escobar J, Bote J, Iskandar A, Tran KV, Tighe DA, Fitzgibbons TP, Aurigemma GP. Comparative left ventricular mechanical deformation in acute apical variant stress cardiomyopathy and acute anterior myocardial infarction utilizing 2-dimensional longitudinal strain imaging. Echocardiography 2020;37:832-40. [Crossref] [PubMed]
- MacIver DH, Partridge JB, Agger P, Stephenson RS, Boukens BJD, Omann C, Jarvis JC, Zhang H. The end of the unique myocardial band: Part II. Clinical and functional considerations. Eur J Cardiothorac Surg 2018;53:120-8. [Crossref] [PubMed]
- Buckberg GD, Hoffman JI, Coghlan HC, Nanda NC. Ventricular structure-function relations in health and disease: Part I. The normal heart. Eur J Cardiothorac Surg 2015;47:587-601. [Crossref] [PubMed]
- Buckberg G. The helical ventricular myocardial band during standard echocardiography: a structure-function relationship. Echocardiography 2015;32:199-204. [Crossref] [PubMed]
- Patrianakos AP, Zacharaki AA, Kalogerakis A, Solidakis G, Parthenakis FI, Vardas PE. Two-dimensional global and segmental longitudinal strain: are the results from software in different high-end ultrasound systems comparable? Echo Res Pract 2015;2:29-39. [Crossref] [PubMed]