Secondary sclerosing cholangitis in a critically ill patient
Introduction
The critically ill are often referred for imaging to evaluate abnormal laboratory values that reflect liver dysfunction. Common etiologies, such as cholecystitis, hepatitis, and vascular thrombosis, are readily recognized given their prevalence and well-known imaging findings. The less well-known though often fatal condition of Secondary Sclerosing Cholangitis, or Sclerosing Cholangitis in Critically Ill Patients (SC-CIP), should be an additional consideration in ICU patients presenting for evaluation. We present a case of a critically ill patient who developed classic clinical and radiologic findings of sclerosing cholangitis in a previously normal native liver during the course of his intensive care unit hospitalization. Our aim is to bring greater attention amongst radiologists to this important and dangerous entity.
Case presentation
A previously healthy 19-year-old male initially presented to an outside facility with progressive fevers, chills, diaphoresis, and headache of five days duration. Per the patient’s family, he had been in his usual state of health prior to the onset of his symptoms and was without any known sick contacts. His brother had recently returned from a 6-week trip to Cambodia and Korea, and was well.
Upon arrival at the emergency department, he was hemodynamically stable though febrile to 101.8 °F and noted to have a systolic murmur and abdominal macular rash. Lab work revealed pancytopenia. Broad spectrum antibiotics were initiated. Initial blood cultures as well as screening for human immunodeficiency virus, mononucleosis, and malaria were all negative. A bone marrow biopsy was performed to evaluate the pancytopenia and was unremarkable. Following the biopsy, the patient ran from the procedure room and fell, hitting his head, and subsequently had a grand mal seizure. A head CT was negative at this time. He was intubated and transferred to a second outside hospital three days following his initial presentation.
At this second hospital, he was nonverbal on examination though would withdraw to stimuli. An extensive laboratory work-up failed to reveal a cause of the seizures, and a lumbar puncture revealed normal cerebrospinal fluid. MRI of the brain revealed a nonspecific right putamen lesion. He was then transferred to our institution on anti-epileptic medications for continuous electroencephalogram (EEG) monitoring and a higher level of care on day 5 following initial presentation.
Upon admission, the patient was nonverbal and not withdrawing to pain. Anti-epileptic medications were continued with noted subsequent decline in epileptic activity on EEG monitoring. Weeks one and two following admission were notable for profound electrolyte abnormalities, felt secondary to central diabetes insipidus, with elevated lactate, creatine kinase, and transaminitis (peak aspartate transaminase or AST of 607 U/L, peak alanine transaminase or ALT of 443 U/L). The patient was hypotensive with a pressor requirement and junctional rhythm, but was without structural cardiac abnormality upon trans-thoracic echocardiography. An extensive infectious work-up, including Herpes Simplex Virus, Epstein-Barr Virus, and West Nile Virus, were negative. A CT head was unremarkable with two separate MRI’s of the brain revealing progressive T2 bright lesions within the bilateral basal ganglia and internal capsules, a nonspecific finding but felt to be reflective of infectious or inflammatory process. Bone marrow biopsy results were consistent with an infectious disease process, but the pathogen remained unknown. Seizure activity was persistent.
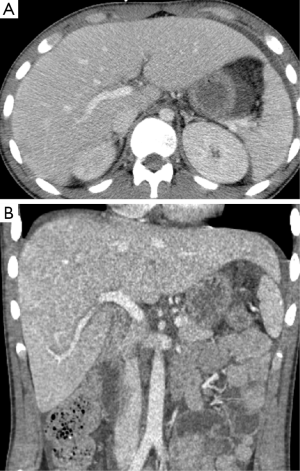
During weeks 3 and 4, two CT’s of the abdomen and pelvis were obtained for neoplastic work-up as well as persistent fevers, and revealed fluid overload with anasarca, pleural effusions, and periportal edema but an otherwise normal liver without biliary dilatation (Figure 1). The patient was noted to be persistently febrile and hypotensive with increasing pressor requirements. The patient developed cardiogenic shock, possibly secondary to volume overload, after which diuresis was initiated. Progressive transaminitis (peak AST 8,530 U/L, peak ALT 3,458 U/L) with newly elevated alkaline phosphatase (peak alkaline phosphatase 258 U/L) and bilirubin (peak total bilirubin or TB 1.6 mg/dL) prompted a right upper quadrant ultrasound which was notable for hepatomegaly, mild biliary dilatation, and elevated hepatic artery velocities felt possibly secondary to increased cardiac output. Because of increasing lactate levels and increasing abdominal distension, an exploratory-laparotomy was performed, which revealed diffusely ischemic though viable bowel. Continuous Renal Replacement Therapy (CRRT) was started, followed by an episode of severe hypotension with high pressor requirements. Subsequently, the patient’s lactate normalized and vasopressor medications were weaned. During hospital week 8, transient mild elevation of AST (peak 107 U/L), ALT (peak 227 U/L), and alkaline phosphatase (peak 1,245 U/L) prompted another abdominal ultrasound, which was notable only for mild hepatomegaly and echogenic kidneys. The patient’s seizures continued to be refractory to treatment despite multiple interventions, with the cause remaining elusive despite brain biopsy. He remained in the critical care unit throughout his hospitalization.
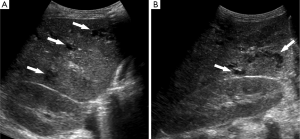
Approximately 4 months following the onset of his symptoms and 3 months following his hypotensive episodes, repeat ultrasound was obtained in the setting of elevated white blood cell count, persistent mild transaminitis, and increasing alkaline phosphatase, now measuring >3,500 U/L (upper limits of laboratory detection). In addition to persistent hepatosplenomegaly, there was new intrahepatic and extrahepatic biliary dilatation and beaded appearance of the intrahepatic bile ducts (Figures 2,3). Sludge was seen within a dilated common bile duct. Within the liver, there were multiple, complex-appearing hypoechoic lesions, concerning for multiple intra-hepatic abscesses in the setting of ascending cholangitis. A CT abdomen/pelvis confirmed the ultrasound findings, revealing over twenty intrahepatic lesions consistent with abscesses. Furthermore, the CT revealed the additional finding of focal areas of narrowing within the biliary tree interspersed with areas of ductal dilatation, similar to the appearance of sclerosing cholangitis (Figure 3).
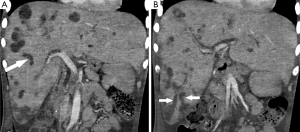
Given the patient’s prolonged hospitalization and intractable seizures, these imaging findings prompted a family meeting, at which the patient was made do-not-resuscitate and then comfort measures only. The patient subsequently passed away, approximately 4.5 months after his initial admission date. The patient’s family declined requests for a post-mortem examination.
Discussion
Sclerosing cholangitis is a cholestatic disorder affecting the intra- and/or extrahepatic biliary tree which, when progressive, leads to biliary cirrhosis and liver failure (1). The primary form of sclerosing cholangitis (PSC) is idiopathic and familiar to radiologists, particular regarding its association with inflammatory bowel disease. Secondary (i.e., acquired) sclerosing cholangitis resulting from ischemic injury to the biliary tree is also well described in the radiology literature as a complication of liver transplantation or hepatic chemoembolization. In recent years, a new type of secondary sclerosing cholangitis has been recognized among critically ill patients with previously normal livers receiving long term intensive care unit treatment. Termed “Sclerosing Cholangitis in Critically Ill Patients (SC-CIP)”, it is a deadly entity with fewer than 100 reported cases in the Critical Care and Gastroenterology literature (2-7), and only a single report in the radiology literature (8). SC-CIP is a disease entity that occurs in critically ill patients with no history of prior liver disease and without a pathological process to explain bile duct obstruction, though is thought to develop in the setting of cholestasis and biliary cast formation (8).
The liver receives blood supply from the portal vein and hepatic arteries, with the biliary epithelium and cholangiocytes supplied solely by the hepatic arteries (9). The arterial supply of the biliary system is composed of a vascular plexus, originating from branches arising directly from the right, left and any accessory hepatic arteries and their segmental branches. Indirect blood supply also originates from the gastroduodenal artery via its distal branches supplying the common bile duct (10). Hypoperfusion of the biliary blood supply can result in poor function or death of the cholangiocytes (11). Damage to the biliary epithelium will both decrease bile flow and also impair cholangiocyte fluid secretion, altering bile composition (12,13). These factors as well as biliary infection, fasting, and hemolysis are all potential factors in biliary cast formation (14).
Biliary cast formation can be seen in patients with any mechanism leading to altered bile clearance, either by increased viscosity or decreased flow (14). To date it has been most commonly seen following orthotopic liver transplantation or chemoembolization for hepatic neoplasm. In orthotopic liver transplant patients, defective bile duct reconstruction or arterial thrombosis can lead to ischemia of the biliary epithelium, promoting decreased flow (14). A similar pathogenesis is seen in patients following transarterial chemoembolization (15,16). Further, systemic infection can alter bile viscosity, upsetting the balance of mucus, calcium bilirubinate and cholesterol crystals, and promote biliary stasis (14).
Secondary sclerosing cholangitis is rare in native, non-transplanted livers, yet as mentioned, recent literature describes secondary sclerosing cholangitis in patients recovering from critical illness in the intensive care unit (1-8). Biliary stasis and sludge is common in the critically ill, particular in patients with neurosurgical intervention or recent abdominal surgery, such as in the presented patient. Fasting and chronic total parenteral nutrition promote gallbladder hypocontractility (14), predisposing to stasis. SC-CIP is characterized by persistent, progressive cholestasis with sclerosing cholangitis on cholangiography (8). Clinical signs typically feature early rapid and marked increase in alkaline phosphatase and gamma-glutamyl transferase with mild increase in serum bilirubin (1,8). Cholestasis is typically persistent, even in cases where there has been resolution of the patient’s primary illness. The presented case is consistent with these findings, with marked elevation in alkaline phosphatase prior to further follow-up imaging.
Current literature suggests that a critical component in the development of SC-CIP is prolonged or severe arterial hypoperfusion of the biliary perivascular plexus in the setting of hypotension, possibly exacerbated by high-dose vasoconstrictors and mechanical ventilation with high positive end-expiratory pressure (PEEP) (1,5). The latter has been shown to alter hepatosplanchnic hemodynamics (17,18). This hypoperfusion leads to biliary cast formation with subsequent biliary infection. The presented case was notable for a period of prolonged hypotension shortly before SC-CIP was diagnosed on imaging, as well as mechanical ventilation with PEEP. Medications currently known to cause cholestasis with bile duct injury or sclerosing cholangitis-like cholestasis (19) were not administered to the patient during his hospitalization.
Imaging findings are helpful for making the diagnosis. Cross-sectional imaging may initially reveal extensive biliary cast formation with associated infections. Subsequent imaging will typically demonstrate multiple irregular strictures, dilatations, beading, and wall thickening of the intra-hepatic biliary ducts, in a pattern similar to that seen in primary sclerosing cholangitis, with relative sparing of the common bile duct (7,8). These findings are consistent with the presented case, which demonstrates biliary strictures, dilatations, and multiple hepatic abscesses as well as debris within the biliary tree on ultrasound and CT. Radiologic differential considerations include ischemic cholangiopathy (typically seen in liver transplant patients), primary sclerosing cholangitis, and other forms of secondary sclerosing cholangitis (though these entities rarely demonstrate biliary cast formation) (8).
Treatment for SC-CIP includes short-term attempt at removal of accessible biliary casts by endoscopy, with liver transplantation being the only current curative option. If liver transplantation is not performed, cirrhosis will develop (1-5). Unfortunately for the patient presented, given his general overall poor prognosis, no attempt was made at these treatment options.
In conclusion, secondary sclerosing cholangitis and biliary cast formation may develop in critically ill patients who have experienced episodes of systemic hypotension, likely due to ischemia of the biliary epithelium. While a familiar entity in the setting of liver transplantation and as a complication of transarterial chemoembolization, SS-CIP is now being recognized as a cause of hepatic failure in native livers in the setting of prolonged intensive care unit stays. Our report is intended to raise awareness of this rare condition and its grave prognosis.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Esposito I, Kubisova A, Stiehl A, Kulaksiz H, Schirmacher P. Secondary sclerosing cholangitis after intensive care unit treatment: clues to the histopathological differential diagnosis. Virchows Arch 2008;453:339-45. [Crossref] [PubMed]
- D'Haens GR, Ruchim MA, Goldberg MJ, Baker AL. Massive intra-hepatic and extra-hepatic bile cast formation after cholecystectomy. Gastrointest Endosc 1993;39:579-81. [Crossref] [PubMed]
- Byrne MF, Chong HI, O'Donovan D, Sheehan KM, Leader MB, Kay E, McCormick PA, Broe P, Murray FE. Idiopathic cholangiopathy in a biliary cast syndrome necessitating liver transplantation following head trauma. Eur J Gastroenterol Hepatol 2003;15:415-7. [Crossref] [PubMed]
- Engler S, Elsing C, Flechtenmacher C, Theilmann L, Stremmel W, Stiehl A. Progressive sclerosing cholangitis after septic shock: a new variant of vanishing bile duct disorders. Gut 2003;52:688-93. [Crossref] [PubMed]
- Gelbmann CM, Rümmele P, Wimmer M, Hofstädter F, Göhlmann B, Endlicher E, Kullmann F, Langgartner J, Schölmerich J. Ischemic-like cholangiopathy with secondary sclerosing cholangitis in critically ill patients. Am J Gastroenterol 2007;102:1221-9. [Crossref] [PubMed]
- Patel KV, Zaman S, Chang F, Wilkinson M. Rare case of severe cholangiopathy following critical illness. BMJ Case Rep 2014;2014.
- Leonhardt S, Veltzke-Schlieker W, Adler A, Schott E, Hetzer R, Schaffartzik W, Tryba M, Neuhaus P, Seehofer D. Trigger mechanisms of secondary sclerosing cholangitis in critically ill patients. Crit Care 2015;19:131. [Crossref] [PubMed]
- Kwon ON, Cho SH, Park CK, Mun SH. Biliary cast formation with sclerosing cholangitis in critically ill patient: case report and literature review. Korean J Radiol 2012;13:358-62. [Crossref] [PubMed]
- Greenway CV, Lautt WW. Hepatic circulation. Comprehensive Physiology, 2011. Available online: http://onlinelibrary.wiley.com/doi/10.1002/cphy.cp060141/abstract;jsessionid=10E6A6EC4EEAEA8B6A0DB6538AE723EC.f01t04?userIsAuthenticated=false&deniedAccessCustomisedMessage=
- Stapleton GN, Hickman R, Terblanche J. Blood supply of the right and left hepatic ducts. Br J Surg 1998;85:202-7. [Crossref] [PubMed]
- Cursio R, Gugenheim J. Ischemia-Reperfusion Injury and Ischemic-Type Biliary Lesions following Liver Transplantation. J Transplant 2012;2012:164329.
- Strazzabosco M, Spirlí C, Okolicsanyi L. Pathophysiology of the intrahepatic biliary epithelium. J Gastroenterol Hepatol 2000;15:244-53. [Crossref] [PubMed]
- Tabibian JH, Masyuk AI, Masyuk TV, O'Hara SP, LaRusso NF. Physiology of cholangiocytes. Compr Physiol 2013;3:541-65. [PubMed]
- Parry SD, Muiesan P. Cholangiopathy and the biliary cast syndrome. Eur J Gastroenterol Hepatol 2003;15:341-3. [Crossref] [PubMed]
- Kim HK, Chung YH, Song BC, Yang SH, Yoon HK, Yu E, Sung KB, Lee YS, Lee SG, Suh DJ. Ischemic bile duct injury as a serious complication after transarterial chemoembolization in patients with hepatocellular carcinoma. J Clin Gastroenterol 2001;32:423-7. [Crossref] [PubMed]
- Hasegawa K, Kubota K, Aoki T, Hirai I, Miyazawa M, Ohtomo K, Makuuchi M. Ischemic cholangitis caused by transcatheter hepatic arterial chemoembolization 10 months after resection of the extrahepatic bile duct. Cardiovasc Intervent Radiol 2000;23:304-6. [Crossref] [PubMed]
- Putensen C, Wrigge H, Hering R. The effects of mechanical ventilation on the gut and abdomen. Curr Opin Crit Care 2006;12:160-5. [Crossref] [PubMed]
- Jakob SM. The effects of mechanical ventilation on hepato-splanchnic perfusion. Curr Opin Crit Care 2010;16:165-8. [Crossref] [PubMed]
- Padda MS, Sanchez M, Akhtar AJ, Boyer JL. Drug-induced cholestasis. Hepatology 2011;53:1377-87. [Crossref] [PubMed]


