Can pontine trigeminal T2-hyperintensity suggest herpetic etiology of trigeminal neuralgia?
Introduction
Trigeminal neuralgia (TN) is characterized by paroxysmal attacks of pain, usually described as lancinating, burning or electric shock-like in the territory of one or more branches of the trigeminal nerve. Attacks usually last only some seconds but may recur repeatedly within a short period of time. They are often precipitated by mild sensory stimulation of the so-called trigger zones, which may be located anywhere within the territory of the affected nerve. Attacks of TN affect commonly the second and third division of the nerve (1).
TN occurs in both genders (with a slight female predominance) and the diagnosis is most common in subjects older than 50, even though young adults and children can also be affected (2).
TN is usually classified into two different categories: primary/idiopathic and secondary (3).
Many cases (80–90%) of idiopathic TN are related to the compression of the sensory nerve root by a vascular structure, most often the superior cerebellar artery (SCA) and are amenable to surgical decompression [neurovascular conflict (NVC)] (4).
Multiple sclerosis (MS) and herpetic infection can be a cause of symptomatic TN, associated to brainstem lesions along the trigeminal descending tract (3).
We have investigated retrospectively the frequency of magnetic resonance imaging (MRI) pontine lesions in patients with TN without MS or brainstem stroke and their association with herpetic infection.
Methods
MRI brain scans of twenty-eight patients (18F, 10M; mean age 52.8±14 years; range of age 26–80 years) with TN without MS or brainstem infarction were retrospectively reviewed by two neuroradiologists with 20 and 24 years of experience, respectively.
Eighteen patients (12F, 6M; mean age 50 years; range of age 26–77 years) had idiopathic TN while ten patients (6F, 4M; mean age 50 years; range of age 34–63 years) had a clinical suspicion of a herpes zoster (HZ) infection; only four of these ten patients performed lumbar puncture that confirmed the diagnosis.
In all cases a brain MRI was performed (Gyroscan Intera, 1.5 T, Philips Medical System, Best, The Netherlands), including FLAIR (TR: 8,005 ms, TE: 100 ms, TI: 2,200 ms, matrix: 256×192, slice thickness: 5 mm), Fast Spin-Echo (FSE) T1- and T2-weighted (TR: 583–4,454 ms, TE: 15–100 ms, matrix: 244×194−384×288, slice thickness: 5 mm, respectively) sequences; axial three-dimensional (3D) FSE T2 with a driven equilibrium pulse (DRIVE) sequence (TE: 250; TR: 1,500; flip angle: 90°; matrix: 256×256; slice thickness: 1×1×1 mm; FOV: 130×130) and magnetic resonance angiography (MRA) were performed to depict NVC and nerve atrophy. MRA was performed without contrast medium injection by using a 3-dimensional time-of-flight sequence (3D TOF, TE: 65 ms; TR: 6.9 ms; flip angle: 20°; matrix: 160×160; slice thickness: 1.40 mm; FOV: 175×200 mm). Multi planar reformation (MPR) and maximum intensity projection (MIP) reconstructions were subsequently analyzed.
We evaluated the presence of brainstem lesions and their correlation with the side of the pain and the TN category.
Results
Seven patients with HZ infection showed hyperintense pontine areas on turbo spin echo (TSE) T2-weighted and FLAIR images, which we called “trigeminal pontine sign” (TPS), all of them without a NVC. Their data were summarized in Table 1. MRI was always consistent with the side of the pain. These lesions had a linear appearance, involving the pathway from the trigeminal root entry zone (REZ) toward the dorso-lateral area of the pons, where the trigeminal tract and the nucleus are located (Figure 1).
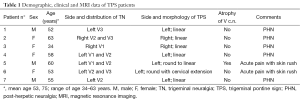
Full table
Fifteen patients (11F, 4M; mean age 50 years; range of age 26–77 years) had a MRI diagnosis of NVC. In twelve of them the sensory nerve root was in contact with the SCA: three had a duplication of the vessel. One NVC patient had a persistent trigeminal artery, while in two patients the NVC was uncertain since it was due to a vein. A bilateral congenital volumetric reduction of the cerebello-pontine cisterns was noted in two cases, associated with a NVC with the s.c.a.
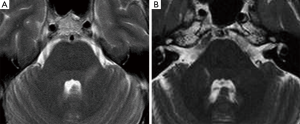
Coronal MPR of T2w DRIVE images showed atrophy of the trigeminal cisternal segment in seven cases: one of them also had the TPS. In two cases a NVC was depicted too, in other four patients the atrophy was isolated and ipsilateral to the side of the pain (Figure 2). We have depicted trigeminal nerve atrophy in four patients with HZ infection.
The remaining two MRI studies were completely normal.
Discussion
The pathophysiology of TN has been much debated, the pain being ascribed variously to hyperactivity or abnormal discharges arising from the Gasserian ganglion, the injured nerve root and the trigeminal nucleus within the brainstem (1,5,6). Important contributing factors may be central and peripheral demyelination, especially in the trigeminal REZ (7).
In our TN series, we found TPS only in patients with clinical suspicion of HZ. TPS are defined as linear hyperintense bands along intrapontine trigeminal fibers. It has been hypothesized that a centripetal migration of varicella-zoster virus (VZV) from the Gasser ganglion to the sensory pontine fibers, aided by the proximity of the ganglion to the REZ, may cause neuronal intra-axial degenerative changes with demyelination, cell death and atrophy (8).
Some articles described these findings in patients with TN. To our knowledge there are only thirteen cases of T2 brainstem hyperintensities reported among patients with TN and related to HZ infection (9-13).
Post herpetic neuralgia (PHN) is the most common neurological sequelae of HZ: it is defined as a pain persisting after 120 days from the onset of the rash. Pain persisting within 30 days is defined as acute herpetic neuralgia, while if persisting between 30 and 120 days is defined as subacute herpetic neuralgia (14,15).
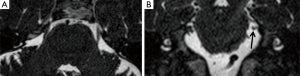
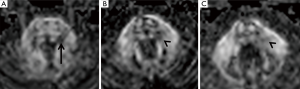
Haanpää et al. (9) found association between MRI brainstem abnormalities and the development of PHN. In our series, acute pain associated with a skin rush was present in only two patients (Table 1). Their MRI revealed different brainstem hyperintense T2 lesions. Patient n°5 (Figure 3A) had a round area located on the left and posterior pontine side, near to the trigeminal nucleus, while patient n°6 had a lesion extending into the brainstem along the whole trigeminal pathway to the cervical spine (Table 1).
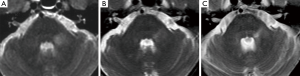
Patient n°6 was lost to follow-up. In patient n°5 MRI performed after 5 years revealed the change from a round to a linear shape of the pontine lesion, extending from the REZ to the trigeminal pontine nucleus (Figure 3B). MRI also depicted a volumetric reduction of the trigeminal cisternal segment, not present earlier. MRI findings worsened at the subsequent follow-up (15 years), revealing an increase in T2 signal hyperintensity and in longitudinal downward extension to the bulbo-pontine junction (Figure 3C). In this setting, also a diffusion tensor imaging (DTI) was performed, showing a reduction of fractional anisotropy (FA) in the left portion of the pons compared to the contralateral side at the level of the REZ and of the trigeminal nucleus (Figure 4). This was probably related to reduction of fiber density and altered myelination.
Conversely, Kidd et al. (10) described a case of HZ with a pontine lesion, attributing its pathogenesis to an episode of shingles but reporting a complete MRI recovery after 4 months.
None of the other five patients developed further lesions in follow-up MRI (mean of 5 years) without changes of the primary TPS.
We may speculate that the evolution of the pathology with the persistence of the lesion along the trigeminal pathway could be crucial in maintaining pain and allodynia.
Other pontine lesions in TN patients, located near to the REZ, are reported in association with ischemic (6,16,17) and demyelinating (MS) (18) pathologies.
Only four cases of TN with linear T2 hyperintensities, similar to our PHN patients, are reported in literature caused by ischemic strokes although related also to a NVC (19).
None of our NVC patients had TPS on MRI even though NVC may induce focal demyelination of the trigeminal nerve root, which is probably involved in the pathogenesis of most cases of TN (20). The difference between HZ and NVC may be related to VZV centripetal migration ability along the sensory pontine fibers inducing demyelination.
Two of our NVC patients also had a congenital volumetric reduction of the cerebello-pontine cisterns. It has been reported that a sharp trigeminal pontine angle may be a useful additional diagnostic factor for idiopathic TN, facilitating the NVC (21,22).
The volumetric reduction of trigeminal nerve is commonly associated with NVC (23-25), while in our series we have depicted it also in PHN patients (4/10). Atrophic changes of the nerve could be secondary to axonal loss and demyelination, probably related not only to the severity of vascular compression but also to the longstanding TN.
Moreover, MRI hyperintensities along the trigeminal nerve roots have been reported in patients with MS (18,26-28). It is known that TN occurs more frequently in MS setting than in general population (18,29). Nakashima et al. (26) described five patients with MS and linear-shape hyperintense lesions similar to our patients, neuro-anatomically corresponding to the intramedullary trigeminal pathway. The author sustained that the trigeminal nerve root might have been previously damaged by herpetic infections and that there is probably a common pathogenetic mechanism in both conditions. There are some experimental evidences (30,31) that, after corneal inoculation, HSV-1 spreads trans-axonally to the central nervous system, through the first branch of the trigeminal nerve, inducing selective demyelination of the intramedullary trigeminal tract. The localization and shape similarities of these lesions suggest that a common pathogenetic mechanism may be also present in PHN patients.
MRI and MRA are often used in the work-up of patients with TN and pontine lesions are rarely seen. Few reports have been published and some uncertainty about the etiology of these findings remained.
Conclusions
Although nonspecific, TPS should be considered an MRI sign of TN, associated with ipsilateral pain and related to PHN.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Love S, Coakham HB. Trigeminal neuralgia: pathology and pathogenesis. Brain 2001;124:2347-60. [Crossref] [PubMed]
- Childs AM, Meaney JF, Ferrie CD, Holland PC. Neurovascular compression of the trigeminal and glossopharyngeal nerve: three case reports. Arch Dis Child 2000;82:311-5. [Crossref] [PubMed]
- Eller JL, Raslan AM, Burchiel KJ. Trigeminal neuralgia: definition and classification. Neurosurg Focus 2005;18:E3. [Crossref] [PubMed]
- Lorenzoni J, David P, Levivier M. Patterns of neurovascular compression in patients with classic trigeminal neuralgia: A high-resolution MRI-based study. Eur J Radiol 2012;81:1851-7. [Crossref] [PubMed]
- Jannetta PJ. Arterial compression of the trigeminal nerve at the pons in patients with trigeminal neuralgia. J Neurosurg 1967;26:159-62. [Crossref] [PubMed]
- Golby AJ, Norbash A, Silverberg GD. Trigeminal neuralgia resulting from infarction of the root entry zone of the trigeminal nerve: case report. Neurosurgery 1998;43:620-2; discussion 622-3. [Crossref] [PubMed]
- Hilton DA, Love S, Gradidge T, Coakham HB. Pathological findings associated with trigeminal neuralgia caused by vascular compression. Neurosurgery 1994;35:299-303; discussion 303. [Crossref] [PubMed]
- Dueland AN, Ranneberg-Nilsen T, Degré M. Detection of latent varicella zoster virus DNA and human gene sequences in human trigeminal ganglia by in situ amplification combined with in situ hybridization. Arch Virol 1995;140:2055-66. [Crossref] [PubMed]
- Haanpää M, Dastidar P, Weinberg A, Levin M, Miettinen A, Lapinlampi A, Laippala P, Nurmikko T. CSF and MRI findings in patients with acute herpes zoster. Neurology 1998;51:1405-11. [Crossref] [PubMed]
- Kidd D, Duncan JS, Thompson EJ. Pontine inflammatory lesion due to shingles. J Neurol Neurosurg Psychiatry 1998;65:208. [Crossref] [PubMed]
- Nagane Y, Utsugisawa K, Yonezawa H, Tohgi H. A case with trigeminal herpes zoster manifesting a long lesion of the spinal trigeminal nucleus and tract on MR T2-weighted image. Rinsho Shinkeigaku 2001;41:56-9. [PubMed]
- Aribandi M, Aribandi L. MRI of trigeminal zoster. Neurology 2005;65:1812. [Crossref] [PubMed]
- Pérez Navarro JM, Escamilla Sevilla F, Pastor Rull J. Trigeminal herpes zoster. Pons hypersignal in magnetic resonance imaging. Neurologia 2007;22:46. [PubMed]
- Nagel MA, Gilden D. Complications of varicella zoster virus reactivation. Curr Treat Options Neurol 2013;15:439-53. [Crossref] [PubMed]
- Jung BF, Johnson RW, Griffin DR, Dworkin RH. Risk factors for postherpetic neuralgia in patients with herpes zoster. Neurology 2004;62:1545-51. [Crossref] [PubMed]
- Kim JS, Kang JH, Lee MC. Trigeminal neuralgia after pontine infarction. Neurology 1998;51:1511-2. [Crossref] [PubMed]
- Delitala A, Brunori A, Chiappetta F. Trigeminal neuralgia resulting from infarction of the root entry zone of the trigeminal nerve: case report. Neurosurgery 1999;45:202. [PubMed]
- Gass A, Kitchen N, MacManus DG, Moseley IF, Hennerici MG, Miller DH. Trigeminal neuralgia in patients with multiple sclerosis: lesion localization with magnetic resonance imaging. Neurology 1997;49:1142-4. [Crossref] [PubMed]
- Arrese I, Lagares A, Alday R, Ramos A, Rivas JJ, Lobato RD. Typical trigeminal neuralgia associated with brainstem white matter lesions on MRI in patients without criteria of multiple sclerosis. Acta Neurochir (Wien) 2008;150:1157-61. [Crossref] [PubMed]
- Love S, Hilton DA, Coakham HB. Central demyelination of the Vth nerve root in trigeminal neuralgia associated with vascular compression. Brain Pathol 1998;8:1-11; discussion 11-2. [Crossref] [PubMed]
- Ha SM, Kim SH, Yoo EH, Han IB, Shin DA, Cho KG, Chung SS, Park YS. Patients with idiopathic trigeminal neuralgia have a sharper-than-normal trigeminal-pontine angle and trigeminal nerve atrophy. Acta Neurochir (Wien) 2012;154:1627-33. [Crossref] [PubMed]
- Parise M, Acioly MA, Ribeiro CT, Vincent M, Gasparetto EL. The role of the cerebellopontine angle cistern area and trigeminal nerve length in the pathogenesis of trigeminal neuralgia: a prospective case-control study. Acta Neurochir (Wien) 2013;155:863-8. [Crossref] [PubMed]
- Erbay SH, Bhadelia RA, O'Callaghan M, Gupta P, Riesenburger R, Krackov W, Polak JF. Nerve atrophy in severe trigeminal neuralgia: noninvasive confirmation at MR imaging--initial experience. Radiology 2006;238:689-92. [Crossref] [PubMed]
- Kress B, Schindler M, Rasche D, Hähnel S, Tronnier V, Sartor K, Stippich C. MRI volumetry for the preoperative diagnosis of trigeminal neuralgia. Eur Radiol 2005;15:1344-8. [Crossref] [PubMed]
- Leal PR, Barbier C, Hermier M, Souza MA, Cristino-Filho G, Sindou M. Atrophic changes in the trigeminal nerves of patients with trigeminal neuralgia due to neurovascular compression and their association with the severity of compression and clinical outcomes. J Neurosurg 2014;120:1484-95. [Crossref] [PubMed]
- Nakashima I, Fujihara K, Kimpara T, Okita N, Takase S, Itoyama Y. Linear pontine trigeminal root lesions in multiple sclerosis: clinical and magnetic resonance imaging studies in 5 cases. Arch Neurol 2001;58:101-4. [Crossref] [PubMed]
- Mills RJ, Young CA, Smith ET. Central trigeminal involvement in multiple sclerosis using high-resolution MRI at 3 T. Br J Radiol 2010;83:493-8. [Crossref] [PubMed]
- Meaney JF, Watt JW, Eldridge PR, Whitehouse GH, Wells JC, Miles JB. Association between trigeminal neuralgia and multiple sclerosis: role of magnetic resonance imaging. J Neurol Neurosurg Psychiatry 1995;59:253-9. [Crossref] [PubMed]
- Hooge JP, Redekop WK. Trigeminal neuralgia in multiple sclerosis. Neurology 1995;45:1294-6. [Crossref] [PubMed]
- Itoyama Y, Sekizawa T, Openshaw H, Kogure K, Goto I. Early loss of astrocytes in herpes simplex virus-induced central nervous system demyelination. Ann Neurol 1991;29:285-92. [Crossref] [PubMed]
- Kristensson K, Vahlne A, Persson LA, Lycke E. Neural spread of herpes simplex virus types 1 and 2 in mice after corneal or subcutaneous (footpad) inoculation. J Neurol Sci 1978;35:331-40. [Crossref] [PubMed]


