Quantitative computed tomography imaging of airway remodeling in severe asthma
Asthma is a chronic inflammatory condition involving the airways that causes increase in bronchial hyperresponsiveness and induces recurrent episodes of wheezing, breathlessness, chest tightness and coughing usually associated with widespread but variable airflow obstruction that is often reversible either spontaneously or after bronchodilator inhalation.
Asthma is also a heterogeneous condition and approximately 5% to 10% of asthmatic subjects have severe disease, characterized by permanent airflow obstruction, frequent exacerbations and hospitalizations, and significant morbidity and mortality (1). Severe asthma is associated with structure changes of the airways that may develop overtime or shortly after onset of disease. These structural changes identified at bronchial biopsies include thickening of reticular basal membrane, hypertrophy and hyperplasia of smooth muscle, mucous hyperplasia, dysregulated extracellular matrix deposition, and increased vasculature (2).
The identification of remodeling pattern in various phenotypes of severe asthma, and the ability to relate airway structures to important clinical outcomes should help target treatment more effectively. As biopsy specimens are limited in size and depth and limited to central airway, and the procedure is too invasive to be repeated, CT imaging may play a role in the noninvasive assessment of airway structure (3-5).
So far, the clinical indication of CT in asthma has been restricted to the identification of associated conditions, such as allergic bronchopulmonary aspergillosis and detection of condition that may mimic asthma, such as hypersensitivity pneumonitis or obliterative bronchiolitis. Qualitative assessment of CT findings in asthma has been performed by a number of studies using HRCT technique (incremental acquisition with 10 mm interval). The findings observed are variably bronchial wall thickening, bronchiectasis, mucus plugging, decreased lung attenuation and gas trapping. In a large cohort of severe asthmatics, Gupta et al. identified bronchial wall thickening and bronchiectasis in 62% and 40% of patients respectively (6).
Quantitative computed tomography (QCT) assessment of large airways in asthma
New QCT imaging has enabled us to study the large airway architecture in detail and assess indirectly the small airway structure. The advent of multidetector row CT and advances in postprocessing techniques have made quantitative assessment of the airway tree and lung parenchyma possible. MDCT technology allows isotropic acquisitions with submillimeter resolution, based on small voxels having almost cubic dimensions (0.35 mm × 0.35 mm × 0.4 mm) over the whole chest during a single breath hold. This isotropic acquisition of data permits multiplanar reformations in any direction into the volume with a high resolution.
Several platforms have been developed for 3D analysis of the proximal airways (7-10). The most recent softwares can be used on thin collimation volumetric acquisition and allow the following steps:
- Airway lumen extraction by automatic registration of the inner surface of the airways;
- Automatic generation of the central line of the tracheobronchial tree;
- Automatic reformations of the airways strictly orthogonal to the central line to minimize errors due to oblique orientations;
- Segmentation of the lumen-wall and wall-lung parenchyma boundaries on the airway reformatted sections;
- Measurements of the airway luminal section area (LA), airway wall section area (WA) and percentage of wall-area (WA%) (Figures 1,2). The registration of the tracheobronchial tree is usually obtained up to 5th or 6th generations.
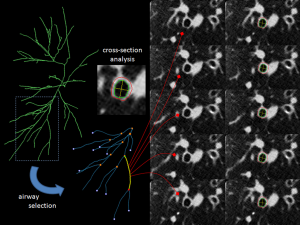
Different algorithms to segment airway wall and lumen have been developed. They were reviewed by Hackx et al. (14). Full width at half maximum (FWHM) is the earliest and simplest algorithm. A seed point is placed in the lumen and rays cast out radially passing from the airway lumen to the wall and then to the lung parenchyma. The point at which the attenuation is half point to maximum on the lumen side marks the inner boundary, and the point of which the attenuation is half way to the local minimum on the parenchymal side marks the outer boundary. These points are connected using an interpolation method to form the inner and outer airway edge. To avoid vessels adjacent to airway, rays that spread into those vessels are manually removed. FWHM however consistently underestimates luminal dimensions and overestimates wall dimensions. These errors increase as the airway generation increases. Some improvements to these algorithms have been proposed with integral based algorithms, which minimize the CT’s blurring effect (15), and “phase congruency approach” which uses multiple reconstruction algorithm to localize airway wall and in order to limit the influence of the reconstruction kernel. Another approach was the Laplacian of Gaussian algorithm which utilizes smoothening and edge detection filters to find abrupt attenuation changes, and provide binary image (16).
The most recent algorithms are based on characterization of each pixel by several parameters such as its attenuation value, its distance from other pixels, or the parameters of the neighboring pixels. Different mathematical morphology functions are then applied permitting to find the ideal paths among pixels corresponding to the airway inner and outer contours (11,17-20). For instance, one of these algorithms based on energy-driven contour estimation (EDCE) consists in a fully automatic segmentation method combining mathematical morphology and deformable contour approach (11,12). These new algorithms have advantage of yielding reliable measurements of a selected airway, regardless of the presence of an adjacent vessel.
Various studies have utilized CT for noninvasive quantitative assessment of proximal airway structural changes in asthmatics. Airway wall thickening in asthma has been shown to correlate with hyperresponsiveness (21-23), airflow limitation (21,22,24,25), gas trapping on expiratory CT (25,26), and asthma control (13).
So far only few studies have demonstrated a correlation between QCT assessed airway remodeling and asthma severity (21,24,27). Aysola et al. prospectively applied QCT analysis of airways in a multicentric cohort of 123 subjects including 63 severe asthmatics, 32 mild-moderate asthmatics, and 25 non-asthmatics. They showed severe asthmatics have thicker airway walls on MDCT than mild asthmatics and normals. The percentage of wall thickness (WT%) or percentage of wall area (WA%) correlated with pathologic measures of remodeling obtained from bronchial biopsies (21).
Airway lumen narrowing is another characteristic of proximal airway morphology in severe asthmatics, demonstrated by QCT assessment. For Gupta et al., the right upper lobe apical bronchus lumen area corrected for body surface area was significantly narrowed in 99 severe asthmatics compared to healthy subjects (22). Brillet et al. studied bronchial lumen area geometry using MDCT in a series of 32 severe asthmatics, 13 mild-moderate asthmatics and pooled controls (28). They showed airway morphologic changes observed in severe asthmatics were characterized by lumen narrowing, wall thickening, and focal bronchial stenoses (Figure 3). Airway narrowing was correlated to airflow obstruction, and two identified clusters of severe asthmatics differed for parameters characterizing airway narrowing (28). In another study, including 65 asthmatics patients and 30 healthy subjects, Gupta et al. identified three clusters of asthmatic patients on the basis of measurement of the right upper lobe apical segmental bronchus (RB1) lumen volume, and RB1 wall volume (29). The results of these three studies suggest that 2D/3D analysis of airway wall volume, luminal narrowing and bronchial stenoses should be regarded as the imaging criteria to assess morphologic remodeling of large airways in severe asthmatics.
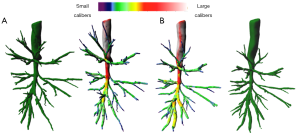
Fetita et al. attempted to improve ability in grading severity in asthma to capture and quantify the airway remodeling process both at the level of the airway wall thickness and airway lumen. Two morphological changes are targeted: (I) the airway wall thickening measured as a global index characterizing the increase of wall thickness above a normal value of wall-to-lumen-radius ratio; and (II) the bronchoarterial ratio index assessed globally from numerous locations in the lungs (Figure 4). The authors showed that the combination of these indices provides a grading of severity of the remodeling process which correlates with the known phenotype of the patients investigated (30,31).
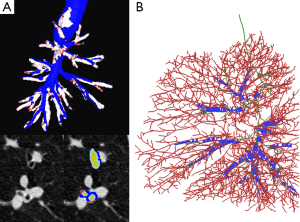
Although CT assessment of proximal airway remodeling has proven to be a sensitive method to detect and quantify changes after administration of treatment (32-35), the reproducibility of measurements between successive acquisitions (interscan variability) needs to be fully established. Brillet et al. showed that the 95% confidence interval between repeated MDCT scans was between –1.59 and –1.5 mm2 for LA and –3.31 and –2.96 mm2 for WA measured on segmental and subsegmental bronchi (36). This variability between CT examinations may impair measurements particularly when small bronchi are considered.
QCT assessment of airway morphology suffers from several sources of variations including CT scanner, algorithms, CT acquisition and reconstruction parameters, particularly field of view (voxel size) (37), inspiration level, patient age and body surface, and the metrix utilized for assessing the airways (14). Body surface area has been used for normalization of airway wall area and lumen area (22,24). Some investigators achieved airway morphometry normalization by calculating airway dimensions of a hypothetic airway with internal perimeter of 10 mm and outer airway perimeter of 20 mm using linear regression from dimensions of all airways measured for each patient (29,38). Lederlin et al. proposed to measure the mean bronchial wall attenuation as a surrogate of wall area, and found better correlation with physiological parameters of airflow limitation compared to other QCT indices (39).
In multicentric trials, recommendations include the use of the same software, the same CT acquisition and reconstruction parameters and the same metrix (3-5). The use of phantoms is also recommended to validate different scanners. The choice of airways to be measured should also be standardized given volumetric measurements of multiple airway should provide more comprehensive assessment.
Small airway disease in asthma
In asthma, the small airways are also affected with significant inflammation and remodeling. Small airway remodeling can be detected on CT scans as indirect changes. The small airways dysfunction results in reduced ventilation of part of the lung which induces reflex vasoconstriction highlighted as area of decreased attenuation on CT images. Heterogeneity of lung attenuation on inspiration CT scans is accentuated in expiratory scans due to regional difference in small airway closure (mosaic perfusion, and gas trapping). In a series by Laurent et al., mosaic perfusion and gas trapping were observed in 23% of moderate persistent asthmatics without significant change in gas trapping scores after inhalation of bronchodilator (40). This allows to exclude the hypothesis of bronchoconstriction to explain gas trapping in these patients, and to reinforce the role of airway remodeling.
Quantitative computed tomography (QCT) assessment of gas trapping in asthma
Extent of low attenuation areas on expiratory CT scans can be quantitatively assessed using the lung densitometry that consists to calculate the % of pixels in lung parenchyma having an attenuation value below a predetermined threshold (Figure 5). Different indices to quantify gas trapping in asthma have been developed including –850 HU attenuation as a threshold at functional residual capacity (26), –900 HU as a threshold at full expiration (41), mean lung density expiratory to inspiratory ratio (25), difference between inspiratory and expiratory lung attenuation (42), and lowest 10th percentile lung attenuation frequency distribution (43). Deformable registration techniques of the inspiration and expiration images have allowed to provide a voxel to voxel ventilation map based on the change in CT attenuation between inspiration and expiration. This technique initially developed to characterize small airway disease in COPD patients allows parametric maps classifying voxels of normal lung, small airway disease, and emphysema (44).
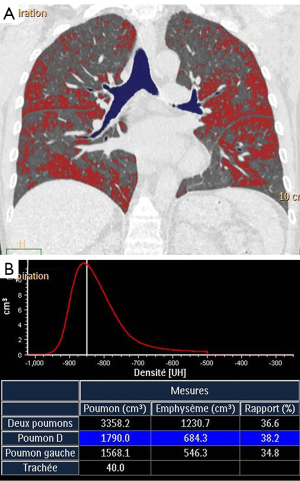
CT assessment of gas trapping in asthma has been associated with airway hyperresponsiveness (41,45), disease duration (26), airflow limitation (25,26) and has been used for evaluation of response to inhaled steroids (42). In addition gas trapping has been shown to correlate with asthma severity. Busacker et al. showed that subjects with gas trapping are significantly more likely to have a history of asthma related hospitalizations, ICU visits and mechanical ventilation (26). Similar to proximal airways assessment, there is a wide variation of scanning protocols and indices of gas trapping utilized by investigators. QCT of gas trapping on low dose CT show important variation on repeat CT scans, regardless of lung volume correction or reproducible breath hold (46). In addition, in multicentric trials, the effects of scanner differences and imaging protocol adherence on quantitative assessment of gas trapping may be problematic. Choi et al. demonstrated improved correlation of gas trapping extent with pulmonary function tests measurements when lung volumes are pneumotachometer controlled (90% VC for inspiration and 20% VC for expiration scan) (47). To circumvent the differences in air calibration between scanners and approaches to handling beam hardening and scatter correction, they proposed a fraction-based method using a fixed air fraction to calculate adjacent thresholds and to use the air attenuation measurements within the tracheal lumen to obtain a subject-specific threshold to quantify gas trapping (47).
Conclusions
Severe asthma is a complex heterogeneous disease with high morbidity and mortality. QCT imaging of airways provides us with an opportunity to extend our understanding of this heterogeneous disease. MDCT imaging has enabled us to study the large airway architecture in detail and assess the small airway structure. These new tools of image analysis should provide better phenotyping of severe asthmatics subjects to improve stratification of patients in clinical trials, and for a given patient to predict mortality and morbidity, to select personalized treatment and assess response to existing and new pharmacological and nonpharmacological therapies. However standardization of methods is still needed to allow utilization of quantitative imaging of airways in multicentric and longitudinal trials.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Wenzel S. Severe asthma in adults. Am J Respir Crit Care Med 2005;172:149-60. [PubMed]
- Benayoun L, Druilhe A, Dombret MC, Aubier M, Pretolani M. Airway structural alterations selectively associated with severe asthma. Am J Respir Crit Care Med 2003;167:1360-8. [PubMed]
- Walker C, Gupta S, Hartley R, Brightling CE. Computed tomography scans in severe asthma: utility and clinical implications. Curr Opin Pulm Med 2012;18:42-7. [PubMed]
- Hartley R, Baldi S, Brightling C, Gupta S. Novel imaging approaches in adult asthma and their clinical potential. Expert Rev Clin Immunol 2015;11:1147-62. [PubMed]
- Walker C, Gupta S, Raj V, Siddiqui S, Brightling CE. Imaging advances in asthma. Expert Opin Med Diagn 2011;5:453-65. [PubMed]
- Gupta S, Siddiqui S, Haldar P, Raj JV, Entwisle JJ, Wardlaw AJ, Bradding P, Pavord ID, Green RH, Brightling CE. Qualitative analysis of high-resolution CT scans in severe asthma. Chest 2009;136:1521-8. [PubMed]
- Tschirren J, Hoffman EA, McLennan G, Sonka M. Segmentation and quantitative analysis of intrathoracic airway trees from computed tomography images. Proc Am Thorac Soc 2005;2:484-7, 503-4. [PubMed]
- Lo P, van Ginneken B, Reinhardt JM, Yavarna T, de Jong PA, Irving B, Fetita C, Ortner M, Pinho R, Sijbers J, Feuerstein M, Fabijańska A, Bauer C, Beichel R, Mendoza CS, Wiemker R, Lee J, Reeves AP, Born S, Weinheimer O, van Rikxoort EM, Tschirren J, Mori K, Odry B, Naidich DP, Hartmann I, Hoffman EA, Prokop M, Pedersen JH, de Bruijne M. Extraction of airways from CT (EXACT'09). IEEE Trans Med Imaging 2012;31:2093-107. [PubMed]
- Fetita C, Ortner M, Brillet PY, Prêteux F, Grenier PA. Morphological-aggregative approach for 3D segmentation of pulmonary airways from generic MSCT acquisitions. In: Proc. of Second International Workshop on Pulmonary Image Analysis - Second International Workshop on Pulmonary Image Analysis in conjunction with MICCAI'09, United Kingdom, 2009;215-26.
- Fetita CI, Prêteux F, Beigelman-Aubry C, Grenier P. Pulmonary airways: 3-D reconstruction from multislice CT and clinical investigation. IEEE Trans Med Imaging 2004;23:1353-64. [PubMed]
- Brillet PY, Fetita CI, Beigelman-Aubry C, Saragaglia A, Perchet D, Preteux F, Grenier PA. Quantification of bronchial dimensions at MDCT using dedicated software. Eur Radiol 2007;17:1483-9. [PubMed]
- Ortner M, Fetita C, Brillet PY, Prêteux F, Grenier P. High-throughput morphometric analysis of pulmonary airways in MSCT via a mixed 3D/2D approach. In: Medical Imaging 2011: Computer-Aided Diagnosis, Proceedings of SPIE (SPIE, Bellingham, WA 2011), 79631R:1-12.
- Brillet PY, Grenier PA, Fetita CI, Beigelman-Aubry C, Ould-Hmeidi Y, Ortner M, Nachbaur G, Adamek L, Chanez P. Relationship between the airway wall area and asthma control score in moderate persistent asthma. Eur Radiol 2013;23:1594-602. [PubMed]
- Hackx M, Bankier AA, Gevenois PA. Chronic obstructive pulmonary disease: CT quantification of airways disease. Radiology 2012;265:34-48. [PubMed]
- Achenbach T, Weinheimer O, Dueber C, Heussel CP. Influence of pixel size on quantification of airway wall thickness in computed tomography. J Comput Assist Tomogr 2009;33:725-30. [PubMed]
- Montaudon M, Berger P, de Dietrich G, Braquelaire A, Marthan R, Tunon-de-Lara JM, Laurent F. Assessment of airways with three-dimensional quantitative thin-section CT: in vitro and in vivo validation. Radiology 2007;242:563-72. [PubMed]
- Saragaglia A, Fetita C, Prêteux F. Assessment of airway remodeling in asthma: volumetric versus surface quantification approaches. Med Image Comput Comput Assist Interv 2006;9:413-20. [PubMed]
- Tschirren J, Hoffman EA, McLennan G, Sonka M. Intrathoracic airway trees: segmentation and airway morphology analysis from low-dose CT scans. IEEE Trans Med Imaging 2005;24:1529-39. [PubMed]
- Kiraly AP, Odry BL, Naidich DP, Novak CL. Boundary-specific cost functions for quantitative airway analysis. Med Image Comput Comput Assist Interv 2007;10:784-91. [PubMed]
- Odry BL, Kiraly AP, Godoy MC, Ko J, Naidich DP, Novak CL, Lerallut JF. Automated CT scoring of airway diseases: preliminary results. Acad Radiol 2010;17:1136-45. [PubMed]
- Aysola RS, Hoffman EA, Gierada D, Wenzel S, Cook-Granroth J, Tarsi J, Zheng J, Schechtman KB, Ramkumar TP, Cochran R, Xueping E, Christie C, Newell J, Fain S, Altes TA, Castro M. Airway remodeling measured by multidetector CT is increased in severe asthma and correlates with pathology. Chest 2008;134:1183-91. [PubMed]
- Gupta S, Siddiqui S, Haldar P, Entwisle JJ, Mawby D, Wardlaw AJ, Bradding P, Pavord ID, Green RH, Brightling CE. Quantitative analysis of high-resolution computed tomography scans in severe asthma subphenotypes. Thorax 2010;65:775-81. [PubMed]
- Niimi A, Matsumoto H, Takemura M, Ueda T, Chin K, Mishima M. Relationship of airway wall thickness to airway sensitivity and airway reactivity in asthma. Am J Respir Crit Care Med 2003;168:983-8. [PubMed]
- Niimi A, Matsumoto H, Amitani R, Nakano Y, Mishima M, Minakuchi M, Nishimura K, Itoh H, Izumi T. Airway wall thickness in asthma assessed by computed tomography. Relation to clinical indices. Am J Respir Crit Care Med 2000;162:1518-23. [PubMed]
- Gono H, Fujimoto K, Kawakami S, Kubo K. Evaluation of airway wall thickness and air trapping by HRCT in asymptomatic asthma. Eur Respir J 2003;22:965-71. [PubMed]
- Busacker A, Newell JD Jr, Keefe T, Hoffman EA, Granroth JC, Castro M, Fain S, Wenzel S. A multivariate analysis of risk factors for the air-trapping asthmatic phenotype as measured by quantitative CT analysis. Chest 2009;135:48-56. [PubMed]
- Little SA, Sproule MW, Cowan MD, Macleod KJ, Robertson M, Love JG, Chalmers GW, McSharry CP, Thomson NC. High resolution computed tomographic assessment of airway wall thickness in chronic asthma: reproducibility and relationship with lung function and severity. Thorax 2002;57:247-53. [PubMed]
- Brillet PY, Debray MP, Golmard JL, Ould Hmeidi Y, Fetita C, Taillé C, Aubier M, Grenier PA. Computed tomography assessment of airways throughout bronchial tree demonstrates airway narrowing in severe asthma. Acad Radiol 2015;22:734-42. [PubMed]
- Gupta S, Hartley R, Khan UT, Singapuri A, Hargadon B, Monteiro W, Pavord ID, Sousa AR, Marshall RP, Subramanian D, Parr D, Entwisle JJ, Siddiqui S, Raj V, Brightling CE. Quantitative computed tomography-derived clusters: redefining airway remodeling in asthmatic patients. J Allergy Clin Immunol 2014;133:729-38.e18.
- Fetita C, Brillet PY, Hartley R, Grenier PA, Brightling C. 3D mapping of airway wall thickening in asthma with MSCT: a level set approach. In: Medical Imaging 2014: Computer-Aided Diagnosis, Proceedings of SPIE Vol. 9035 (SPIE, Bellingham, WA 2014), 90352I:1-9.
- Fetita C, Brillet PY, Brightling C, Grenier PA. Grading remodeling severity in asthma based on airway wall thickening index and bronchoarterial ratio measured with MSCT. In: Medical Imaging 2015: Image-Guided Procedures, Robotic Interventions, and Modeling Proceedings of SPIE 2015;9415:941515-22.
- Kurashima K, Kanauchi T, Hoshi T, Takaku Y, Ishiguro T, Takayanagi N, Ubukata M, Sugita Y. Effect of early versus late intervention with inhaled corticosteroids on airway wall thickness in patients with asthma. Respirology 2008;13:1008-13. [PubMed]
- Lee YM, Park JS, Hwang JH, Park SW, Uh ST, Kim YH, Park CS. High-resolution CT findings in patients with near-fatal asthma: comparison of patients with mild-to-severe asthma and normal control subjects and changes in airway abnormalities following steroid treatment. Chest 2004;126:1840-8. [PubMed]
- Niimi A, Matsumoto H, Amitani R, Nakano Y, Sakai H, Takemura M, Ueda T, Chin K, Itoh H, Ingenito EP, Mishima M. Effect of short-term treatment with inhaled corticosteroid on airway wall thickening in asthma. Am J Med 2004;116:725-31. [PubMed]
- Haldar P, Pavord ID, Shaw DE, Berry MA, Thomas M, Brightling CE, Wardlaw AJ, Green RH. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med 2008;178:218-24. [PubMed]
- Brillet PY, Fetita CI, Capderou A, Mitrea M, Dreuil S, Simon JM, Prêteux F, Grenier PA. Variability of bronchial measurements obtained by sequential CT using two computer-based methods. Eur Radiol 2009;19:1139-47. [PubMed]
- Sheshadri A, Rodriguez A, Chen R, Kozlowski J, Burgdorf D, Koch T, Tarsi J, Schutz R, Wilson B, Schechtman K, Leader JK, Hoffman EA, Castro M, Fain SB, Gierada DS. Effect of reducing field of view on multidetector quantitative computed tomography parameters of airway wall thickness in asthma. J Comput Assist Tomogr 2015;39:584-90. [PubMed]
- Kaminska M, Foley S, Maghni K, Storness-Bliss C, Coxson H, Ghezzo H, Lemière C, Olivenstein R, Ernst P, Hamid Q, Martin J. Airway remodeling in subjects with severe asthma with or without chronic persistent airflow obstruction. J Allergy Clin Immunol 2009;124:45-51.e1-4.
- Lederlin M, Laurent F, Portron Y, Ozier A, Cochet H, Berger P, Montaudon M. CT attenuation of the bronchial wall in patients with asthma: comparison with geometric parameters and correlation with function and histologic characteristics. AJR Am J Roentgenol 2012;199:1226-33. [PubMed]
- Laurent F, Latrabe V, Raherison C, Marthan R, Tunon-de-Lara JM. Functional significance of air trapping detected in moderate asthma. Eur Radiol 2000;10:1404-10. [PubMed]
- Newman KB, Lynch DA, Newman LS, Ellegood D, Newell JD Jr. Quantitative computed tomography detects air trapping due to asthma. Chest 1994;106:105-9. [PubMed]
- Tunon-de-Lara JM, Laurent F, Giraud V, Perez T, Aguilaniu B, Meziane H, Basset-Merle A, Chanez P. Air trapping in mild and moderate asthma: effect of inhaled corticosteroids. J Allergy Clin Immunol 2007;119:583-90. [PubMed]
- Goldin JG, McNitt-Gray MF, Sorenson SM, Johnson TD, Dauphinee B, Kleerup EC, Tashkin DP, Aberle DR. Airway hyperreactivity: assessment with helical thin-section CT. Radiology 1998;208:321-9. [PubMed]
- Galbán CJ, Han MK, Boes JL, Chughtai KA, Meyer CR, Johnson TD, Galbán S, Rehemtulla A, Kazerooni EA, Martinez FJ, Ross BD. Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nat Med 2012;18:1711-5. [PubMed]
- Ueda T, Niimi A, Matsumoto H, Takemura M, Hirai T, Yamaguchi M, Matsuoka H, Jinnai M, Muro S, Chin K, Mishima M. Role of small airways in asthma: investigation using high-resolution computed tomography. J Allergy Clin Immunol 2006;118:1019-25. [PubMed]
- Mets OM, Isgum I, Mol CP, Gietema HA, Zanen P, Prokop M, de Jong PA. Variation in quantitative CT air trapping in heavy smokers on repeat CT examinations. Eur Radiol 2012;22:2710-7. [PubMed]
- Choi S, Hoffman EA, Wenzel SE, Castro M, Lin CL. Improved CT-based estimate of pulmonary gas trapping accounting for scanner and lung-volume variations in a multicenter asthmatic study. J Appl Physiol (1985) 2014;117:593-603. [PubMed]