T1 characteristics of interstitial pulmonary fibrosis on 3T MRI—a predictor of early interstitial change?
Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic and progressive restrictive lung condition with unknown aetiology causing irreversible damage (1). The long-term outcome is poor and is associated with high mortality (2). There are very few measures available, if any, to diagnose pulmonary fibrosis (PF) or monitor disease progression. Standard diagnostic techniques include lung function tests and imaging with high-resolution computed tomography (HRCT) or histopathology from lung biopsy (2). Typically the lungs display subpleural and paraseptal fibrosis and areas of honeycombing (cystic areas filled with mucin and inflammatory cells) adjacent to areas of unaffected lung parenchyma (so-called spatial heterogeneity). HRCT exposes patients to ionising radiation and allows diagnosis in only around 50% of patients with IPF (3) and has limited accuracy in monitoring disease progress or response to treatment. In fact, lung function tests appear to be the only investigation recommended for monitoring IPF according to current UK guidelines (4). There is therefore a need for more sensitive imaging technique that can identify evolving fibrosis for better risk stratification and monitoring disease progression, and response to treatment.
T1 mapping (relaxometry) evaluates T1 (longitudinal) relaxation times in each image voxel (5). The rate of relaxation is influenced by the physical properties of the tissue (e.g., fibrosis, protein deposition or oedema) and therefore a study of relaxation phenomena (T1 mapping) can investigate these properties (6). Factors that influence the T1 value include: the compound in which the hydrogen nuclei are found (e.g., water or lipids etc.), the surrounding chemicals (in particular, the amount of water binding to non-water hydrogen-containing compounds and the amount of free water) and the presence of gadolinium that reduces T1 values (7,8). T1 mapping has been used to assess myocardial fibrosis (9,10) and is reported to allow more precise quantification of area of infracted myocardium (11). Previous studies in patients with severe aortic stenosis showed strong correlation between native T1 and myocardial fibrosis as assessed by myocardial biopsy following aortic valve replacement (12,13).
The aim of this study was to evaluate T1 signal characteristics in the fibrotic lung parenchyma T1 at different time points (pre- and post-administration of Gadolinium) and to investigate the feasibility of the technique in prediction of early fibrotic lung changes that may not be visible on computed tomography (CT).
Methods and materials
The local ethics committee approved the study and written informed consent was obtained. Patients with known IPF as confirmed by imaging or histopathology were selected from the University of Edinburgh interstitial lung diseases (ILD) database. Exclusion criteria included: glomerular filtration rate <60 mL/1.73 m2, history of previous reaction to gadolinium, patients who had received gadolinium-based contrast 3 months prior to recruitment, pregnancy or breast feeding, pacemakers and defibrillators, critical illness, patients receiving ventilator support, and respiratory comorbidities (e.g., pulmonary embolism, emphysema and lung cancer). Seven patients with IPF underwent a repeat magnetic resonance imaging (MRI) scan 7–18 days later to test reproducibility of results.
The control group included healthy volunteers (n=10) with no known medical history of smoking, PF or conditions leading to PF (including systemic sclerosis, rheumatoid arthritis, and drugs with known fibrotic lung side effects). Control subjects were primarily recruited from a group of healthy volunteers undergoing myocardial T1 mapping studies and through advertisement.
Only subjects with known fibrosis underwent HRCT scan and MRI on the same day of attendance. Normal subjects underwent MRI only. MR imaging was performed on a 3T system (Verio, Siemens AG, Healthcare Sector, Erlangen, Germany). Thoracic CT scans were performed on a 320-multidetector row CT scanner (Aquilion ONE, Toshiba Medical Systems, Nasushiobara, Japan). Low dose scout imaging was performed to localise the thoracic structures. The scan was planned to image the whole thorax from lung apices to lowest borders of the lungs (0.5-mm collimation). Tube voltage was selected based on body mass index and tube current was selected using automated software (SureExposure, Toshiba Medical Systems). Toshiba’s iterative reconstruction algorithm (Adaptive Iterative Dose Reduction 3D; AIDR-3D, strong) was used to reconstruct 0.5-mm sections from the raw data (Toshiba Medical Systems, Nasushiobara, Japan).
All patients and controls underwent multi-echo T1-weighted MRI using the modified look-locker inversion-recovery (MOLLI; 124×192 acquisition matrix; 8 mm slice) recovery technique to measure signal intensity at different inversion times (T1). The imaging was performed during a 15–20 s breathhold in a single slice (flip angle 35°, echo time 1 ms, repetition time 740 ms, inversion times 100, 180, 260, 850, 913, 993, 1,583, 1,646, 1,743, 2,476 and 3,226 ms). The positioning of the image slice was determined by HRCT, where there was visual evidence of PF. MOLLI imaging was performed prior to the contrast administration, and at 15, 25, 30 and 35 min post Gadolinium (administration of 0.1 mmoL/kg gadobutrol (Gadovist, Bayer Pharma AG, Berlin, Germany). MOLLI imaging in controls was only performed before and 10 min after contrast injection due to the restrictions of the myocardial T1 mapping protocol.
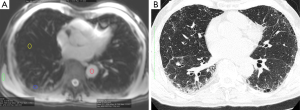
Regions of interest (ROI) were drawn on the MOLLI images in fibrotic lung tissue and morphologically normal lung tissue, as guided by corresponding HRCT findings (Figure 1). Additional regions of interests (ROIs) were drawn in the descending aorta and thoracic wall skeletal muscle. T1 was estimated for each ROI by nonlinear fitting of the MOLLI signal equation (14) using Matlab (The Mathworks, Natick, MA, USA). Estimated T1 values derived from data for which the recovery curve fit no better than a horizontal line [as determined by calculating the Akaike information criterion for each fit (15) were excluded from analysis as this indicated imprecise data]. As a result, the following numbers of T1 values were excluded: 2 (total ROI =78) in fibrotic lung, 16 in normal lung of IPF patients (total ROI =67), and 9 T1 values from controls were excluded (total ROI =17).
Data was evaluated using SPSS v19 (SPSS Inc., Chicago, IL, USA) and MedCalc statistical software (v9.6) were used for all statistical analysis. Data was tested for normality of distribution with Shapiro-Wilks or non-parametric Levene’s test as appropriate. For comparison of ROI mean signal intensity data and average T1 values of fibrotic and morphologically normal lung independent 2-tailed Student’s t-test or 2-tailed Mann-Whitney U test were used as appropriate. For comparison of T1 values of fibrotic or normal lung between first scan with second scan 2-tailed paired Student’s t-test or Wilcoxon signed rank test were used as appropriate. For comparison of T1 values of fibrotic or morphologically normal lung across acquisition times one-way ANOVA or Kruskall-Wallis test were used. The mean pre-contrast T1 value of skeletal muscle in IPF patients in the repeated scans were plotted using a Bland-Altman analysis (16) to check for agreement in the means between the two scans. Statistical significance was defined as P<0.05. Data is expressed or displayed as the mean ±1.96 standard error of mean unless otherwise stated.
Results
Ten patients (M:F, 8:2) with mean age of 69.9 (+10.3) years and ten healthy volunteers (M:F, 8:5) with mean age of 68.8 (+14.4) years were recruited.
T1 values of skeletal muscle of IPF patients and control subjects yielded an estimated pre-contrast T1 value of 1,078±62 and 1,109±78 ms, respectively (P value>0.35; Mann-Whitney U test). There was no significant statistical difference in pre-contrast T1 value of skeletal muscle in IPF patients in the repeated scans (P value>0.99; 2-tailed paired Student’s t-test) and the level of agreement by Bland-Altman analysis was considered good (5.7% observed variation; Figure 2).
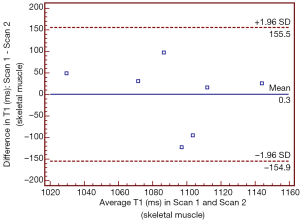
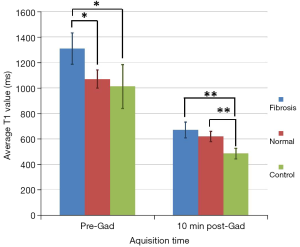
Table 1 and Figure 3 summarise the differences in T1 values of fibrotic and non-fibrotic lung in patients and controls before and after administration of Gadolinium. Before gadolinium administration, the T1 value of fibrotic lung tissue was significantly higher than that of normal appearing lung tissue from patients with IPF and control subjects (ANOVA, P=0.02). Pre-gadolinium T1 value of morphologically normal lung tissue from patients was not significantly different from that of controls. Ten min after gadolinium administration the T1 value of fibrotic and normal lung in IPF patients was significantly greater than that of normal lung tissue in controls (Kruskall-Wallis test, P=0.001).

Full table
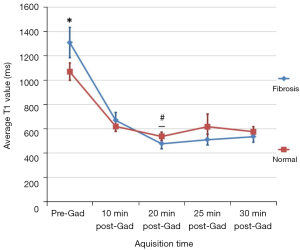
T1 for fibrotic lung continued to decrease until 20 min after contrast agent administration (P≤0.0001), whereas morphologically normal lung T1 did not significantly change after 10 min (P>0.3; Table 1). The T1 values of fibrotic lung was significantly lower than normal lung in IPF patients from 20 min post Gadolinium administration (P<0.05; Figure 4).
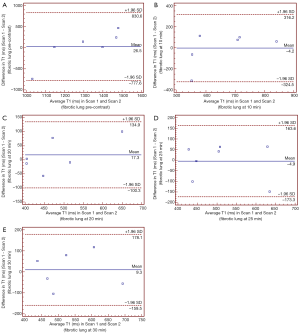
Bland-Altman plots for T1 values in fibrotic lungs (pre- and post-contrast) are shown in Figure 5. This shows good reproducibility for the estimation of T1 values with all cases remain within the limits of agreement. The mean difference (bias) between T1 levels was relatively small.
Discussion
In this feasibility study with limited sample size, we tested the feasibility and reproducibility of T1 mapping at 3T in identification of established and potentially progressive PF that is not yet visible on CT.
In this study, the T1 values of pre-contrast skeletal muscle in this study were similar to the previously published value at 3T of 1,072±10 ms using a shortened MOLLI recovery protocol (17). Acceptable reproducibility of the T1 values in the fibrotic lung was observed when comparing the results of the repeated scans. The difference may be explained by variations in the slice position between studies resulting in different ROI values.
This study shows that the fibrotic lung has a higher pre-contrast T1 than either morphologically normal lung or control lung. Morphologically normal lung T1 in IPF patients and control lung T1 were not significantly different pre-contrast. However, at 10 min after administration of Gadolinium, control lung had a significantly shorter T1 than either fibrotic or morphologically normal lung. T1 for fibrotic lung continued to decrease until 20 min after contrast agent administration, whereas morphologically normal lung T1 did not significantly change after 10 min.
The differences in T1 values in fibrotic and non-fibrotic lung can be explained by differences in the proton density and changes in tissue composition. There was no significant difference between T1 values of the normal lung with control lung. This may show the similarity in pre-contrast T1 values, or the limited sensitivity of the technique to identify the differences. Ten min post-contrast, the reduction in T1 of control lung was significantly different to the T1 of the normal and fibrotic lung. Continuous reduction in T1 in fibrotic lung compared to normal lung in IPF patients indicates continuous uptake of contrast agent in the fibrotic lung after 20 min, compared with morphologically normal lung. Previous studies demonstrated delayed gadolinium enhancement in the fibrotic myocardium due to slower diffusion and increased retention of contrast material in the fibrotic tissue (9,18). The normal tissue is expected to display a greater change in T1 value soon after gadolinium administration and a quicker return to normal T1 values compared to fibrotic tissue.
The above findings indicate that whilst the differences in fibrotic and normal lung in IPF patients can be identified on pre-contrast T1 mapping, Gadolinium administration is necessary to identify early fibrotic changes (not yet detectable by CT) from truly normal lung.
There are a number of limitations to this study. Firstly, limited sample size might have potentially resulted in selection bias. It should also be appreciated that the radiological features of IPF are varied and the study population was heterogeneous; some patients had predominantly ground-glass disease and others having predominantly honeycombing disease. Given the differences in pathology, the T1 value of these two tissue types may be different. Averaging all T1 values of fibrotic tissue masks any difference and does not truly reflect the spectrum of changes in IPF. Unfortunately this study population was too small to allow sub-categorisation of fibrotic tissue, thus future studies could assess if there is a difference in T1 values.
Stadler et al. (19) demonstrated that the inspirational status in healthy volunteers during 1.5 T MR examinations affects T1 values; higher T1 is expected on expiration than inspiration. Moreover ventral lung T1 values were significantly lower than dorsal lung T1 values, probably due to pooling of blood in the supine position. All images in this study were obtained in full inspiration, which may have reduced the proton density within ROIs compared to full expiration, thus full inspiration may have produced a lower signal-to-noise ratio resulting in a poor fit in some cases, especially in the normal lung. It may be worthwhile to conduct future studies with full expiration. Comparison of T1 values between different subject may be interpreted with some degree of caution, due to potential differences related to the degree of inspiration and the position of ROIs (e.g., anterior versus posterior lung). In addition, the enhanced T2* effect at 3T would have resulted in reduced SNR which further improved following the administration of Gadolinium.
A potential source of T1 estimation error in this study is respiratory motion (when the patient was not able to hold his breath) and cardiac motion. Application of breathing navigation and ECG gating techniques may reduce the effect of motion. Image registration may also reduce the effect of motion.
In this pilot study we used a single slice imaging technique. This limited the number of ROIs drawn to acquire T1 measurements. In future validation studies, multiple breathholds will be performed to image the whole lung and measure T1 in multiple ROIs within the lungs.
In summary, this pilot study has shown that T1 mapping of patients with IPF at 3T is feasible and may demonstrate significant difference between fibrotic lung tissue and morphologically normal lung tissue in these IPF patients, and may is able to evaluate early fibrosis in patients with apparently morphologically normal lung. Larger cohorts with long-term follow-up will be necessary to confirm the validity of above statement.
Acknowledgements
This work is supported by a research grant (CHSS Exec. 19.01.12) from Chest, Heart, Stroke Scotland.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- King TE Jr, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet 2011;378:1949-61. [PubMed]
- Nicholson AG, Colby TV, du Bois RM, Hansell DM, Wells AU. The prognostic significance of the histologic pattern of interstitial pneumonia in patients presenting with the clinical entity of cryptogenic fibrosing alveolitis. Am J Respir Crit Care Med 2000;162:2213-7. [PubMed]
- Souza CA, Müller NL, Flint J, Wright JL, Churg A. Idiopathic pulmonary fibrosis: spectrum of high-resolution CT findings. AJR Am J Roentgenol 2005;185:1531-9. [PubMed]
- National Institute for Health and Care Excellence: Clinical Guidelines. Diagnosis and Management of Suspected Idiopathic Pulmonary Fibrosis: Idiopathic Pulmonary Fibrosis. London: Royal College of Physicians (UK), 2013.
- Carneiro AO, Vilela GR, de Araujo DB, Baffa O. MRI Relaxometry: Methods and Applications. Brazil J Phys 2006;36:9-15.
- Moon JC, Messroghli DR, Kellman P, Piechnik SK, Robson MD, Ugander M, Gatehouse PD, Arai AE, Friedrich MG, Neubauer S, Schulz-Menger J, Schelbert EB; Society for Cardiovascular Magnetic Resonance Imaging; Cardiovascular Magnetic Resonance Working Group of the European Society of Cardiology. Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson 2013;15:92. [PubMed]
- Gowland PA, Stevenson VL. The Longitudinal Relaxation Time. In: Tofts P, editor. Quantitative MRI of the Brain: Measuring Changes Caused by Disease. Chichester, UK: John Wiley & Sons, Ltd, 2003:111-42.
- Caillé JM, Lemanceau B, Bonnemain B. Gadolinium as a contrast agent for NMR. AJNR Am J Neuroradiol 1983;4:1041-2. [PubMed]
- Burt JR, Zimmerman SL, Kamel IR, Halushka M, Bluemke DA. Myocardial T1 mapping: techniques and potential applications. Radiographics 2014;34:377-95. [PubMed]
- Sparrow P, Messroghli DR, Reid S, Ridgway JP, Bainbridge G, Sivananthan MU. Myocardial T1 mapping for detection of left ventricular myocardial fibrosis in chronic aortic regurgitation: pilot study. AJR Am J Roentgenol 2006;187:W630-5. [PubMed]
- Kali A, Choi EY, Sharif B, Kim YJ, Bi X, Spottiswoode B, Cokic I, Yang HJ, Tighiouart M, Conte AH, Li D, Berman DS, Choi BW, Chang HJ, Dharmakumar R. Native T1 Mapping by 3-T CMR Imaging for Characterization of Chronic Myocardial Infarctions. JACC Cardiovasc Imaging 2015;8:1019-30. [PubMed]
- Lee SP, Lee W, Lee JM, Park EA, Kim HK, Kim YJ, Sohn DW. Assessment of diffuse myocardial fibrosis by using MR imaging in asymptomatic patients with aortic stenosis. Radiology 2015;274:359-69. [PubMed]
- Bull S, White SK, Piechnik SK, Flett AS, Ferreira VM, Loudon M, Francis JM, Karamitsos TD, Prendergast BD, Robson MD, Neubauer S, Moon JC, Myerson SG. Human non-contrast T1 values and correlation with histology in diffuse fibrosis. Heart 2013;99:932-7. [PubMed]
- Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med 2004;52:141-6. [PubMed]
- Akaike H. A New Look at the Statistical Model Identification. IEEE Trans Autom Control 1974;19:716-23.
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307-10. [PubMed]
- Falque C, Kober F, Bernard M, Jacquier A. Intersite reproducibility of myocardial assessment of T1 and partition coefficient on phantom and one volunteer at 1.5T and 3.0T. J Cardiovasc Magn Reson 2014;16:79. [PubMed]
- Moon JC, Sheppard M, Reed E, Lee P, Elliott PM, Pennell DJ. The histological basis of late gadolinium enhancement cardiovascular magnetic resonance in a patient with Anderson-Fabry disease. J Cardiovasc Magn Reson 2006;8:479-82. [PubMed]
- Stadler A, Jakob PM, Griswold M, Barth M, Bankier AA. T1 mapping of the entire lung parenchyma: Influence of the respiratory phase in healthy individuals. J Magn Reson Imaging 2005;21:759-64. [PubMed]


