Antral gastritis caused by Helicobacter pylori infection in the pediatric age group is associated with increased mesenteric lymph node dimension observed by ultrasonography
Introduction
Since the discovery of Helicobacter pylori (HP), understanding of gastroduodenal pathology has completely changed (1). HP is the most prevalent gastric microbial pathogen (2). Almost half of the world’s population is estimated to be infected with HP, and it is a major risk factor for several gastroduodenal diseases, including gastric and duodenal ulcer, gastric mucosa-associated lymphoid tissue lymphoma, and gastric cancer (3). A variety of methods for the detection of HP have been described shortly after the identification of this pathogen and they have been continually improved and extended over time (4). Both invasive and noninvasive diagnostic methods are used for HP infection. Although several diagnostic tests are available for the detection of HP infection, all of them have both advantages and disadvantages. The invasive tests require upper gastrointestinal endoscopy for obtaining the diagnostic sample, while non-invasive methods are not totally reliable (4,5).
The antrum is usually the most common site of inflammation and submucosal layer is frequently colonized by HP. Gastric wall thickening is one of the most important signs of gastrointestinal diseases radiologically. Attributed to the mucosal erosion ensourcing from the proliferation of HP, mucosal layer is supposed to become thicker. Similarly, submucosal layer as well as mucosal layer (together with muscularis mucosa) may gain thickness in parallel to the extent and severity of inflammatory changes (6). Up to now, the clinical applications of transabdominal gastric ultrasonography (US) in HP diagnosis have been limited. As a diagnostic tool, US are a non-invasive, safe, cheap and practical option for imaging stomach. A systematic and dynamic approach with awareness of common technical difficulties must be performed by the radiologist to determine the essential clues for the diagnosis (7).
The aim of this prospective study was to find out if transabdominal US might have a predictive role for detection of antral gastritis and HP infection in the antrum of pediatric age group.
Materials and methods
Study design
This study was approved by the local Institutional Review Board. Written informed consent was obtained from all subjects. A total of 91 (63.6%) patients and 52 (36.4%) controls, referred to the department of radiology of our tertiary center, between September 2013 and March 2014, were allocated into two groups:
- Group 1 (n=91): pediatric patients with complaints and endoscopic findings consistent with gastritis and documented HP infection;
- Group 2 (n=52): pediatric patients with complaints and endoscopic findings consistent with gastritis in the absence of documented HP infection.
Diagnoses of antral gastritis and HP were confirmed by the evaluation of biopsies obtained during endoscopic examination. Exclusion criteria were previous history of prior abdominal surgery and/or abdominal radiotherapy, known or suspected diagnosis of abdominal malignancy or inflammatory bowel disease, previous treatment for HP infection, and simple obesity patients (BMI ≥25).
US was routinely performed prior to endoscopy in all cases by an examiner who is unaware of the patient’s clinical features and institutional fasting guidelines (solid and fluid intake up to 8 hours before US examination) were applied. A linear array, high-frequency (6-MHz) probe transducer (Toshiba Aplio 500, Toshiba Medical Systems Corporation, Tokyo, Japan) by appropriate focus and depth settings is used as the abdominal wall is thinner in children than in adults. Patients were examined in supine position followed by the right lateral decubitus position. All measurements were performed from the anterior gastric wall. The transducer was applied on the epigastric region in a sagittal plane. The gastric antrum and body were examined by shifting the transducer from right to left in order to achieve a qualitative impression of the gastric cavity. The antrum has a characteristic multilayered wall and is best visualized in a parasagittal plane just right of the midline using the left lobe as an acoustic window. It is surrounded by the left lobe and caudate lobe of the liver anteriorly and the head or neck of the pancreas posteriorly. The wall thickness is measured in a cross section with a longitudinal section of the superior mesenteric artery in the image. In the transabdominal sonography, a hyperechogen mucosa layer can be observed in the form of linear stripes in an empty stomach and in trabeculated form due to gastric piliforms when there is content in stomach lumen. Right below this layer a HP presence at 80-90% rate can be observed. Outside lies hyperechogen submucosa and hypoechogen muscularis propria and at the outermost section a hyperechogen serosa layer can be observed. However, this outermost hyperechogen mucosa layer was not measured in the present study due to the possibility of being affected by gastric inflammation (Figures 1,2).
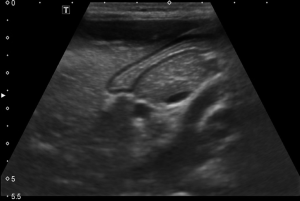
Outcome parameters
Two groups were compared in terms of demographics, clinical, and ultrasonographic parameters such as biggest mesenteric lymph node detected, muscularis mucosa thickness, submucosal thickness, muscularis propria thickness, and total gastric wall thickness.
Statistical analyses
Data were analyzed using the Statistical Package for Social Sciences 21.0 for Windows (SPSS Inc., Chicago, IL, USA). A normal distribution of the univariate data was checked using Kolmogorov-Smirnov test, Shapiro-Wilk test, and coefficient of variation. Parametric tests were applied to data of normal distribution and non-parametric tests were applied to data of questionably normal distribution. Independent-samples t-test and Mann-Whitney U (Exact) test were used to compare independent groups. To calculate correlation coefficients Spearman’s rho test was used. The distribution of categorical variables in both groups was compared using Pearson chi-square test. Data are expressed as mean ± SD or median (interquartile range), as appropriate. All differences associated with a chance probability of 0.05 or less were considered statistically significant.
Results
A total of 91 (63.6%) patients and 52 (36.4%) controls were included in the study. Mean age of the study group was 164.4±36.8 (range, 61-228) months.
Two groups were compared in terms of demographic, clinical, and ultrasonographic parameters. The study groups exhibited no statistically significant difference with respect to age (P=0.747), and presenting symptoms (P=0.982) (Table 1).

Full table
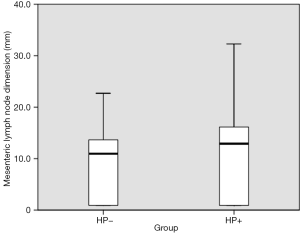
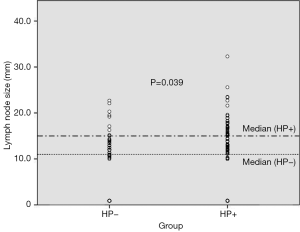
As for the ultrasonographic parameters, mesenteric lymph node dimension was significantly increased in Group 1 (P=0.039). Median mesenteric lymph node dimension was 12.9±15.4 mm in Group 1, while 11.0±12.8 mm in Group 2 (Figure 3). The scatter lot of the lymph node size of the two groups was also shown in Figure 4. No significant difference was observed between groups in terms of muscularis mucosa thickness (P=0.243), submucosal thickness (P=0.174), muscularis propria thickness (P=0.356), and total gastric wall thickness (P=0.223) (Table 1).
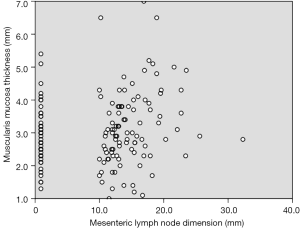
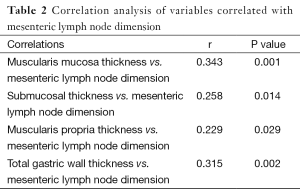
Correlation analysis of variables revealed that muscularis mucosa thickness (r=0.343, P=0.001), submucosal thickness (r=0.258, P=0.014), muscularis propria thickness (r=0.229, P=0.029), and total gastric wall thickness (r=0.315, P=0.002) were positively correlated with mesenteric lymph node dimension (Table 2). Correlation of muscularis propria and mesenteric lymph node dimension was shown in Figure 5.
Full table
Discussion
In this study, we aimed to present novel ultrasonographic tips, which may be useful for diagnosis of antral gastritis and HP infection in the pediatric population. We hope that diagnosis will be easier and cheaper after validation and popularization of these clues.
HP infection can be diagnosed by several methods. Upper gastrointestinal endoscopy should be preferred for cases with a family history of HP infection, cases with symptoms persisting for more than 6 months and affecting activities of daily living (8,9). The interest in noninvasive methods to identify HP continues. In children, the efficacy of the diagnostic tests based on the detection of HP-specific antibodies in blood and body secretions is debatable due to the low sensitivity (10). Okuda et al. evaluated the accuracy of two IgG antibodies tests and revealed sensitivity and specificity of 91.9% and 96.9% for urine-HpELISA and 78.4% and 100% for rapid urine-HpAb (11). Molecular methods are also used for the detection of HP in gastric biopsies. Ou et al. stated that the fluorescent quantitative PCR test was more sensitive than conventional methods (12). A nested PCR had a sensitivity of 93.0% and a specificity of 100% compared with the (13) C-urea breath test on gastric DNA in asymptomatic children (13). Baskovich et al. detected high number of new patients with HP by PCR, suggesting that PCR could be useful in detecting asymptomatic cases (3). In the present study, we aimed to find out ultrasonographic clues, which may be useful for diagnosis of antral gastritis and HP infection in the pediatric population. Our study is unique and original, since we suggest that increase in mesenteric lymph node dimensions may be predictive parameters for detection of antral gastritis and HP infection in US. Cut-off values cannot be established yet due to overlap values, and further controlled studies are necessary for validation and standardization of these parameters. However, we think that our findings will contribute to the improvement of diagnostic capacity of US in antral gastritis and HP infection.
In children, symptoms of HP-related peptic ulcer disease are nonspecific and may include epigastric pain especially after meals, night-time waking, unexplained nausea and/or vomiting, anorexia, hematemesis and iron deficiency anemia (14). HP infection is the most important cause of primary duodenal ulcers in children. Since duodenal ulcer and use of nonsteroidal anti-inflammatory drugs are the most common aetiologies of upper gastrointestinal bleeding during childhood, HP infection should be sought in children with acute upper gastrointestinal bleeding (15). There are several studies investigating the association between recurrent abdominal pain and HP infection, but no association has been identified (16,17). In the present study, the main presenting symptoms were dyspepsia and stomachache.
The main limitations of the present study were the relatively small number of patients and the absence of a control group. The lack of satisfactory data about US findings in the pediatric age group may explain the absence of control group in the present study. In addition, some details of history and factors that may influence the outcome may not be completely documented. Since we only measured the GI wall in Caucasians in a single city of our country, the reference values may not be transferrable to a population with other diets or ethnicities. Furthermore, format of expressing average measurement data for the two groups may not allow for the determination of threshold values and a true positive rate or a true negative rate. Due to these restrictions, associations should be interpreted with caution. However, this article raises awareness of “clues” and suggests pioneering further studies on this method.
Conclusions
In conclusion, our results suggest that, although the P is only 0.039, antral gastritis caused by HP infection in the pediatric age group may be associated with mesenteric lymph node dimension observed by US. Data obtained from this study may serve as baseline information with which to compare findings of gastric US in pediatric patients with HP colonization and antral gastritis. Our findings may be useful in the diagnosis of gastritis pediatric patients and unnecessary interventions and measures can be avoided in some cases. However, further trials on larger series are necessary for making more precise interpretations.
Acknowledgements
The English in this document has been checked by at least two professional editors, both native speakers of English.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1984;1:1311-5. [PubMed]
- Kato S, Sherman PM. What is new related to Helicobacter pylori infection in children and teenagers? Arch Pediatr Adolesc Med 2005;159:415-21. [PubMed]
- Baskovich B, Sun L, Patel R, Wakefield D, Yuan Y, Liu C. Helicobacter pylori detection by polymerase chain reaction in pediatric gastritis. J Pediatr Gastroenterol Nutr 2012;54:698. [PubMed]
- Dzierzanowska-Fangrat K, Lehours P, Mégraud F, Dzierzanowska D. Diagnosis of Helicobacter pylori infection. Helicobacter 2006;11 Suppl 1:6-13. [PubMed]
- Pourakbari B, Ghazi M, Mahmoudi S, Mamishi S, Azhdarkosh H, Najafi M, Kazemi B, Salavati A, Mirsalehian A. Diagnosis of Helicobacter pylori infection by invasive and noninvasive tests. Braz J Microbiol 2013;44:795-8. [PubMed]
- Swenson DW, Wallach M. Helicobacter pylori-associated antral gastritis and ulcer disease: imaging by computed tomography and ultrasound. Ultrasound Q 2012;28:185-7. [PubMed]
- Cubillos J, Tse C, Chan VW, Perlas A. Bedside ultrasound assessment of gastric content: an observational study. Can J Anaesth 2012;59:416-23. [PubMed]
- Guariso G, Meneghel A, Dalla Pozza LV, Romano C, Dall'Oglio L, Lombardi G, Conte S, Calacoci M, Campanozzi A, Nichetti C, Piovan S, Zancan L, Facchin P. Indications to upper gastrointestinal endoscopy in children with dyspepsia. J Pediatr Gastroenterol Nutr 2010;50:493-9. [PubMed]
- Hidaka N, Nakayama Y, Horiuchi A, Kato S, Sano K. Endoscopic identification of Helicobacter pylori gastritis in children. Dig Endosc 2010;22:90-4. [PubMed]
- Alarcón T, José Martínez-Gómez M, Urruzuno P. Helicobacter pylori in pediatrics. Helicobacter 2013;18 Suppl 1:52-7. [PubMed]
- Okuda M, Kamiya S, Booka M, Kikuchi S, Osaki T, Hiwatani T, Maekawa K, Fukuda Y. Diagnostic accuracy of urine-based kits for detection of Helicobacter pylori antibody in children. Pediatr Int 2013;55:337-41. [PubMed]
- Ou Z, Xiong L, Li DY, Geng L, Li L, Chen P, Yang M, Zeng Y, Zhou Z, Xia H, Gong S. Evaluation of a new fluorescence quantitative PCR test for diagnosing Helicobacter pylori infection in children. BMC Gastroenterol 2013;13:7. [PubMed]
- Goncalves MH, Silva CI, Braga-Neto MB, Fialho AB, Fialho AM, Queiroz DM, Braga LL. Helicobacter pylori virulence genes detected by string PCR in children from an urban community in northeastern Brazil. J Clin Microbiol 2013;51:988-9. [PubMed]
- Ertem D. Clinical practice: Helicobacter pylori infection in childhood. Eur J Pediatr 2013;172:1427-34. [PubMed]
- Houben CH, Chiu PW, Lau JY, Lee KH, Ng EK, Tam YH, Yeung CK. Duodenal ulcers dominate acute upper gastrointestinal tract bleeding in childhood: a 10-year experience from Hong Kong. J Dig Dis 2008;9:199-203. [PubMed]
- Spee LA, Madderom MB, Pijpers M, van Leeuwen Y, Berger MY. Association between helicobacter pylori and gastrointestinal symptoms in children. Pediatrics 2010;125:e651-69. [PubMed]
- Mourad-Baars P, Hussey S, Jones NL. Helicobacter pylori infection and childhood. Helicobacter 2010;15 Suppl 1:53-9. [PubMed]