Keratoameloblastoma or Kerato-odontoameloblastoma: report of its soft tissue recurrence with literature review
Introduction
The term keratoameloblastoma (KA) was first proposed in 1958 by Pindborg and Weinmann (1). In 1970, Pindborg described an unusual type of ameloblastoma consisting partly of keratinizing cysts and partly of tumor papilliferous appearance and suggested the term papilliferous KA. Later, described by WHO [1992] as an acanthomatous ameloblastoma (AA) with extensive areas of keratinization (2).
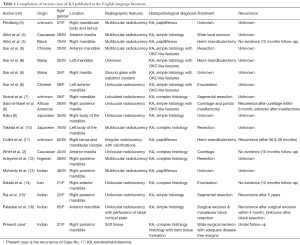
To the best of our knowledge, only 18 cases have been described in the English language literature, out of which only three cases have been reported from the Indian sub-continent (Table 1) (3-16). This case is presented due to its rarity (soft tissue recurrence), and to lay emphasis on the need to differentiate it from other keratin producing intraosseous neoplasms (KPION), with review of literature.
Full table
Case report
A 27-year-old female reported with a complaint of slow growing swelling in the lower right back region of the jaw since 6 to 7 months. Initially there was no pain but later she experienced mild pain during mastication. Patient’s family history was noncontributory. She was apparently alright 5 years back, and then developed a swelling in the same region which was diagnosed as KA. She was operated for the same in our institute. En-block resection of the mandible was preformed followed by reconstruction using titanium plate. She was on regular follow-up and after a period of 3 years, iliac crest graft was placed.
The patient now presented with the facial asymmetry due to the swelling on the right side. It was approximately of 8 cm × 6 cm in its greatest diameter, which extended anterior-posteriorly, from corner of the mouth to the tragus of the ear; superior-inferiorly, below the zygomatic arch to angle of the mandible. The swelling was oval in shape, cystic, fluctuant, non-tender, non-pulsating in nature. The overlying skin was normal with no increase in temperature, and was also not fixed to the surrounding underlying structures. Her mouth opening was also restricted (2 cm). No other extra-oral abnormality was detected. Intra-oral examination also revealed a non-tender swelling of 5 cm × 4 cm in diameter involving the right posterior mandibular (edentulous area) and floor of the mouth. Swelling was obliterating the lingual vestibule posterior to mandibular right second molar and also showed the presence of indentation (upper right molars). Her oral hygiene was fair with few stains and calculus. Axial computed tomography scans revealed soft tissue tumor mass on right side with distinct borders, infiltrating into the masseter muscle (arrow marked). Involvement of superficial lobe of parotid gland was questionable. Tumor also showed infiltration into the medial pterygoid, mylohyoid and to some extent to the hyoglossus muscles, with indistinct borders (Figure 1A). Based on the history, clinical and radiographic examination a differential diagnosis of benign connective tissue neoplasm i.e., rhabdomyoma, leiomyoma and benign salivary gland neoplasm (pleomorphic adenoma) were considered, and a provisional diagnosis of recurrent ameloblastoma was made.
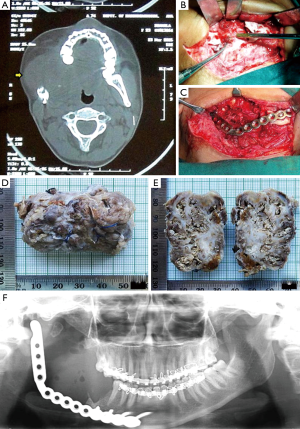
Incisional biopsy confirmed the diagnosis of recurrent KA. The patient was taken under general anesthesia for resection of the lesion. Inter-maxillary fixation was done to achieve the occlusion before placing the incision. A 10 cm long sub-mandibular incision was given and layer-wise dissection was done to expose the bone. Tumor mass was observed on the lingual side of the previously grafted bone (iliac crest). The tumor was exposed by osteotomizing the bone (Figure 1B). At one place the mandibular bone seemed to be eroded by the tumor. Eroded part of previously grafted iliac crest was resected, and the tumor was removed in toto (entire tumor mass in one piece), with adequate disease free margins. During the process of resection the lesion got punctured and showed dark brown colored discharge. The previous reconstruction plate along with diseased bone was removed and a new 2.5 mm SS reconstruction plate with condylar implant was placed, which was secured with four 12 mm × 2.5 mm long screws (Figure 1C). Hemostasis was achieved and wound was closed in layers.
Gross specimen received was oval in shape, brownish in color, firm in consistency and measured about 5.5 cm × 4 cm × 2.5 cm in greatest diameter (Figure 1D). Cut surface revealed numerous cystic spaces with creamish brown cheesy to solid material (Figure 1E,F).
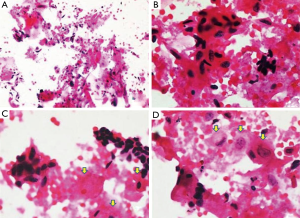
Imprint cytology showed numerous cells, having oval to slightly elongated hyperchromatic nucleus with sparse cytoplasm (odontogenic origin) arranged solitary or in aggregates (Figure 2A,B). Few polygonal cells having round to oval nucleus with abundant cytoplasm (squamous cell) were also appreciated (Arrow marked). Background stroma consists of flakes of keratin-like material within the sea of RBCs (Figure 2C,D). Hence, cytological findings were also suggestive of KA.
The tissue was routinely processed; serial sections were taken, and stained with hematoxylin and eosin (H&E). Histopathological examination revealed many areas of proliferating odontogenic epithelium in the form of follicle, nests, chords, plexiform pattern with multiple cystic spaces (Figure 3A,B). Odontogenic epithelium showed luminal papillary projections (Figure 3C,D) with tall columnar peripheral cells and stellate reticulum-like cells showing squamous metaplasia (Figure 3E,F). Extensive keratin production was appreciated within these islands (Figure 3F), in the connective tissue stroma without inflammatory reaction, and also within the cystic spaces “Keratin filled cystic spaces” (Figure 3G,H). Many areas showed presence of “Pacinian corpuscle-like stacks of lamellated keratin” (Figure 3I,J) and “hair-like appearance” (Figure 3K). Areas adjacent to epithelium showed homogenous eosinophilic areas resembling dentinoid-like material (Figure 3L,M) and few with dentinal tubules (Figure 3N). The background stroma was moderately collagenized with patchy distribution of inflammatory infiltrate and focal areas of cholesterol cleft formation was also observed (Figure 3O).
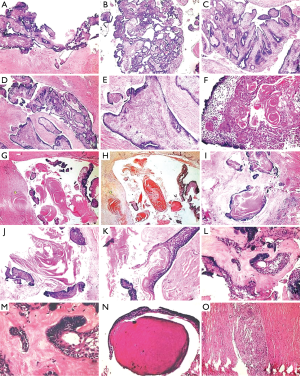
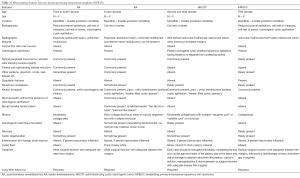
Based on these features, histopathological differential diagnosis of Solid Variant of Keratinizing Cystic Odontogenic Tumor (SKCOT), AA and Keratinizing Primary Intraosseous Squamous Cell Carcinoma (KPISCC) were considered (Table 2), and a final diagnosis of “KA complex histology/Kerato-odontoameloblastoma” was made.
Full table
Discussion
Ameloblastoma is a true neoplasm of enamel organ-type, tissue which does not undergo differentiation to the point of enamel formation. It has been described by Robinson as a benign tumor that is usually “unicentric, non-functional, intermittent in growth, anatomically benign, and clinically persistent” (17).
Ameloblastomas can undergo different forms of metaplasia, and are therefore highly polymorphic benign odontogenic tumors, giving rise to various histological variants like acanthomatous, granular cell, desmoplastic, basal cell, and clear cell ameloblastoma. The cause or the stimulus for this metaplasia is unknown; however, it is generally attributed to the multipotentiality of odontogenic epithelium (13). Formation of keratin in odontogenic tumor is a hallmark histopathological feature of AA, and is well recognized and documented (17). It results due to the squamous metaplasia of the stellate reticulum cell inside the odontogenic follicles, and can even further progress to keratin pearl formation (2). Another variant of ameloblastoma which shows extensive keratinization is KA, first proposed by Pindborg in 1958 (1). In 1992, World Health Organization (WHO) loosely defined KA as an ameloblastoma with an extensive keratin formation (18), though the recent WHO classification for Odontogenic Tumor has not mentioned this term. WHO accepted this lesion within the histological spectrum of AA due to keratinization (19). Norval et al. also suggested that the KA should be considered as a variant of AA (7).
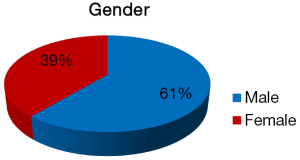
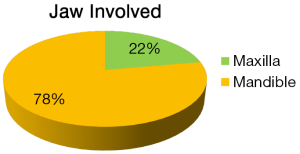
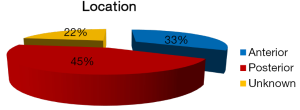
The clinical and histopathological features of 18 reported cases of KA are summarized in Table 1. Most of the patients are from Indian origin followed by Caucasian, Japanese, Malaya, Chinese origin etc. Their age ranged from 21 to 76 years, with a mean age of 42.3 years (fourth decade), and showed male predominance (Figure 4). It frequently involved lower jaw (Figure 5), with an affinity towards the posterior region i.e., body of the mandible & ramus (Figure 6). Although, availability of its clinical data is limited and it usually presents as a symptomatic swelling. Radiographically, majority showing multilocular radiolucency, few showed mixed radiographic pattern, indistinct margins or having ground glass appearance. Literature search also revealed wide variation in its histomorphologic features, and has been classified into four groups by Whitt et al., 2007 (2).
- Papilliferous histology: odontogenic epithelium shows papillary projections into the cystic spaces;
- Simple histology: odontogenic follicles are filled with parakeratin or ortho-keratin and lined by ameloblast-like cells showing reversal of polarity;
- Simple histology with KCOT-like features: shows similar features of simple type, in addition it contains features of conventional KCOT;
- Complex histology: consists of epithelial follicles packed with parakeratin or orthokeratin, extrusion of keratin masses into connective tissue stroma in the form of “Pacinian-like stacks” with or without foreign body reaction.
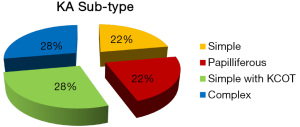
KA shows enormous amount of keratin production, parakeratinization being the most frequently observed as compared to orthokeratinization. It differs from AA, which also exhibits squamous metaplasia of the stellate reticulum (with or without keratin production), by the presence of abundant keratin formation, within the connective tissue stroma (12). Description and comparison of similar other KPION’s is summarized in Table 2. Out of 18 reported cases (Figure 7); 5 cases each of complex KA & Simple KA with KCOT-like features were observed followed by simple KA & papilliferous KA (4 cases each).
In KA, there is proliferation of ameloblastic epithelium in the form of follicles, showing keratinization with or without cystic areas. The initial case reported by Pindborg and Weinmann consisted partly of keratinizing cysts and partly of tumor islands with papilliferous appearance (1). Later, Altini et al. described a similar tumor that did not show the papilliferous epithelium nor the extensive necrosis and debris in the follicles (5). The basis of papilliferous nature of the epithelium seems to be intercellular adherence and different rates of necrosis of individual cells. Separation of the necrotic cells from the remainder epithelium results in the formation of numerous pseudopapillary structures which project into the lumina of the cystic follicles (13). Cases lacking convincing histological evidence of typical ameloblastoma i.e., reversal of polarity has also been reported (5).
Brannon RB observed mural nodules of ameloblastoma as direct proliferations from the KCOT lining (20). Two widely opposed schools of thought have been proposed to explain the evolution of KA; (I) keratinizing ameloblastoma de novo and (II) ameloblastromatous transformation in a preexisting KCOT (2,21,22). A popular belief is that KCOT and ameloblastoma are distinct from each other (23). However, it would seem probable that very occasionally, KCOT may provide the source of epithelium from which ameloblastoma can arise, because the epithelial lining of KCOT shares both phenotype (ameloblastic basal layer) and gene profile (expression of enamel matrix and odontogenic ameloblast-associated proteins) with ameloblast-lineage cells (24,25). Siar and Ng reported the presence of KCOT-like features in the cystic elements of the tumor, and suggested it to be of “hybrid nature” (6). Others have advocated the inclusion of tumors with such histology into the spectrum of KA (8).
Few cases of KA have been described having a complex histomorphology with parakeratin packed epithelial follicles along with “Ribbons of epithelium” showing “Lamellar arrangement of keratin” forming “Hair-like structures” (10) or “Pacinian-like stacks” (2,12). Extrusion of keratin into the fibrous connective tissue stroma is generally seen with or without associated foreign body reaction. Presence of keratin in the stroma could be due to the extensive production & packing of the keratin within the follicles, and subsequently leading to the rupture and its extrusion into the stroma.
It is a known fact that ameloblastoma do not undergo differentiation to the point of enamel formation, and is grouped under “odontogenic epithelium without odontogenic ectomesenchyme” (17). The present case showed presence of ameloblastic epithelium, enormous amount of keratin production, epithelium-mesenchymal induction (hyalinization) with variable amount of mineralization (dentinoid-like material). Authors have also reported cribriform or solid areas with squamous metaplasia, tubular structures, focal granular cell change, cementum or bone-like calcifications and necrotic debris within cystic lumina in KA (2,10,11). Sah K suggests that such a tumor (KA-like features with odontogenic hard tissue formation) can not be characterized as any subtype of ameloblastoma and should be termed as “Kerato-odontoameloblastoma (KOA)”. Based on the present reported case and literature review, Sah K would also like to propose a modification in the classification initially suggested by Whitt et al., 2007 (2).
- Papilliferrous histology;
- Simple histology;
- Simple histology with keratinizing cystic odontogenic tumor (KCOT)-like features;
- Complex histology:
- With odontogenic hard tissue formation “kerato-odontoameloblastoma”;
- Without odontogenic hard tissue formation.
Presence of rapid mitotic rate (2.5/high-power field) has also been related with the biological potential of KA. Occurrence of necrosis and its recurrent behavior justify its classification as a carcinoma, and asserted that the papilliferous KA represents a papillary ameloblastic carcinoma (2).
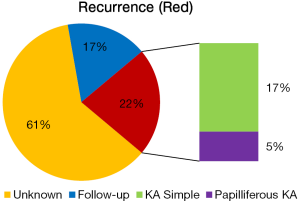
Various treatment modalities have been described in the literature i.e., curettage, enucleation, segmental resection, hemi-mandibulectomy, and even its post-treatment follow-up information is limited (Table 1). It was observed from the existing literature (18 cases of KA) that 21% cases of KA (n=18) showed recurrence, out of which 3 cases are of simple KA and one case of papilliferous KA (Figure 8). Though, local or distant metastasis or death from KA is not still reported.
The present case was initially (5 years back), diagnosed as KA (simple histology), which showed intraosseous involvement of the posterior mandible, and radiographically appeared as a unilocular radiolucency (17). The patient reported after 5 years with recurrence in the soft tissue where the primary lesion was located.
Conclusions
Keratoameloblastoma is a very rare histopathological variant of ameloblastoma, still searching for its identity. Histopathological diagnosis of KA is exceptionally essential to differentiate it with other histopathologically similar KPION, particularly when handling and reporting from small biopsy specimen. Literature search revealed only handful of cases showing substantial variation, lacking proper documentation and follow-up. Such interesting cases should be carefully evaluated & recorded for the better understanding of its diverse clinical, radiological & histomorphologic nature, in order to classify them accordingly, and to formulate appropriate treatment modalities to curtail its recurrence.
Acknowledgements
We would like to acknowledge Prof. (Dr) Alka D. Kale, Dean, VK KLE Institute of Dental Sciences, KLE University and Dr. Vijayalakshmi S. Kotrashetti, Maratha Mandal’s NGH Institute of Dental Sciences and Research Centre, Belgaum, Karnataka, India for their valuable comments and suggestions.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Pindborg JJ, Weinmann JP. Squamous cell metaplasia with calcification in ameloblastomas. Acta Pathol Microbiol Scand 1958;44:247-52. [PubMed]
- Whitt JC, Dunlap CL, Sheets JL, Thompson ML. Keratoameloblastoma: a tumor sui generis or a chimera? Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007;104:368-76. [PubMed]
- Pindborg JJ. Pathology of the dental hard tissues. Philadelphia(PA): W. B. Saunders;1970:381-2.
- Altini M, Lurie R, Shear M. A case report of keratoameloblastoma. Int J Oral Surg 1976;5:245-9. [PubMed]
- Altini M, Slabbert HD, Johnston T. Papilliferous keratoameloblastoma. J Oral Pathol Med 1991;20:46-8. [PubMed]
- Siar CH, Ng KH. ‘Combined ameloblastoma and odontogenic keratocyst’ or ‘keratinising ameloblastoma’. Br J Oral Maxillofac Surg 1993;31:183-6. [PubMed]
- Norval EJ, Thompson IO, van Wyk CW. An unusual variant of keratoameloblastoma. J Oral Pathol Med 1994;23:465-7. [PubMed]
- Said-al-Naief NA, Lumerman H, Ramer M, Kopp W, Kringstein GJ, Persenchino F, Torno R. Keratoameloblastoma of the maxilla. A case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1997;84:535-9. [PubMed]
- Kaku T. Keratoameloblastoma of the mandible. J Oral Pathol Med 2000;29:350.
- Takeda Y, Satoh M, Nakamura S, Ohya T. Keratoameloblastoma with unique histological architecture: an undescribed variation of ameloblastoma. Virchows Arch 2001;439:593-6. [PubMed]
- Collini P, Zucchini N, Vessecchia G, Guzzo M. Papilliferous keratoameloblastoma of mandible: a papillary ameloblastic carcinoma: report of a case with a 6-year follow-up and review of the literature. Int J Surg Pathol 2002;10:149-55. [PubMed]
- Adeyemi B, Adisa A, Fasola A, Akang E. Keratoameloblastoma of the mandible. J Oral Maxillofac Pathol 2010;14:77-9. [PubMed]
- Mohanty N, Rastogi V, Misra SR, Mohanty S. Papilliferous keratoameloblastoma: an extremely rare case report. Case Rep Dent 2013;2013:706128.
- Ketabi MA, Dehghani N, Sadeghi HM, Shams MG, Mohajerani H, Azarsina M, Azizi A. Keratoameloblastoma, a very rare variant of ameloblastoma. J Craniofac Surg 2013;24:2182-6. [PubMed]
- Raj V, Chandra S, Bedi RS, Dwivedi R. Keratoameloblastoma: Report of a rare variant with review of literature. Dent Res J (Isfahan) 2014;11:610-4. [PubMed]
- Palaskar SJ, Pawar RB, Nagpal DD, Patil SS, Kathuriya PT. Keratoameloblastoma a rare entity: a case report. J Clin Diagn Res 2015;9:ZD05-7. [PubMed]
- Shafer WG, Hine MK, Levy BM. A Textbook of Oral Pathology. 4th edition. Philadelphia, Pa, USA: WB Saunders, 1983.
- Kramer IRH, Pindborg JJ, Shear M. World Health Organization international histological classification of tumors: histological typing of odontogenic tumors. 2nd edition. New York: Springer Verlag, 1992.
- Gardner DG, Heikinheima K, Shear M, Philipsen HP, Coleman H. World Health Organization Classification of Tumours: Pathology of Head and Neck Tumours. 1st edition. Lyon, France: IARC Press, 2005.
- Brannon RB. The odontogenic keratocyst. A clinicopathologic study of 312 cases. Part II. Histologic features. Oral Surg Oral Med Oral Pathol 1977;43:233-55. [PubMed]
- Kratochvil F, Stewart J, Kleinegger C. Keratoameloblastoma and solid variant of keratocystic odontogenic tumor. OOOOE 2011;111:e44.3.
- Sisto JM, Olsen GG. Keratoameloblastoma: complex histologic variant of ameloblastoma. J Oral Maxillofac Surg 2012;70:860-4. [PubMed]
- Eversole LR, Sabes WR, Rovin S. Aggressive growth and neoplastic potential of odontogenic cysts: with special reference to central epidermoid and mucoepidermoid carcinomas. Cancer 1975;35:270-82. [PubMed]
- Gold L. Biologic behaviour of ameloblastoma. Oral Maxillofac Surg Clin North Am 1991;3:21-30.
- Ren C, Amm HM, DeVilliers P, Wu Y, Deatherage JR, Liu Z, MacDougall M. Targeting the sonic hedgehog pathway in keratocystic odontogenic tumor. J Biol Chem 2012;287:27117-25. [PubMed]