Current role of multiparametric magnetic resonance imaging for prostate cancer
Introduction
Currently, the diagnostic pathway for prostate cancer detection is initiated on prostate-specific antigen (PSA) level and digital rectal exam (DRE). Use of PSA as a screening tool followed by systematic transrectal ultrasound (TRUS)-guided biopsy has resulted in increased detection of prostate cancer with stage migration toward low-risk disease. About 233,000 new prostate cancers are estimated to be diagnosed in 2014 in the USA (1). This has come with the risk of overdiagnosis and overtreatment, as many of these are clinically insignificant low-risk prostate cancer. On the other hand, anterior tumors tend to be missed by TRUS biopsy until they grow to a substantial size and reach within 15-20 mm from the posterior margin of the prostate, leading to delayed diagnosis. Systematic TRUS biopsy has historically shown to underestimate the final Gleason grade of tumor on histology following radical prostatectomy, leading to inaccurate risk stratification and selection of therapeutic options. For all these reasons, the US and the Canadian Task Force on Preventive Health Care recently released independent statements arguing that the risks of PSA tests outweigh the benefits (2).
Multiparametric magnetic resonance imaging (mp-MRI), combining the morphological assessment of T2-weighted imaging (T2WI) with diffusion-weighted imaging (DWI), dynamic contrast-enhanced imaging (DCEI) perfusion and magnetic resonance spectroscopic imaging (MRSI), has been extensively studied in recent years (3-6). In particular, T2WI and DWI have shown considerable promise in the detection, localization, risk stratification and staging of prostate cancer (7-13). This review will provide an overview of the different imaging sequences and discuss the current role of mp-MRI in the different aspects of management of prostate cancer.
Multiparametric MRI technique
Currently, mp-MRI is regarded as the reference standard imaging modality for prostate cancer because a single MRI sequence cannot adequately detect and characterize prostate cancer. Although the ideal set of sequences for prostate mp-MRI has not been determined, mp-MRI is composed of high-resolution T2WI, DWI, and DCEI with optional MRSI (Figures 1-3) (13-15). T1-weighted Imaging (T1WI) is of limited use in assessing prostate morphology or in identifying tumor within the gland. Its main use is in detecting post-biopsy hemorrhage. Bowel motion artifacts should be reduced by administering anti-peristaltic agents. Prostate imaging at 3T benefits from higher signal to noise ratio (SNR). Use of endorectal coil (ERC) is not an absolute requirement for cancer detection protocol, but is preferable at 1.5T (14). ERC use is recommended for staging purposes, although patient acceptability and increased costs remain its drawbacks. The principle, strong and weak points of each mp-MRI sequence are summarized in Table 1.
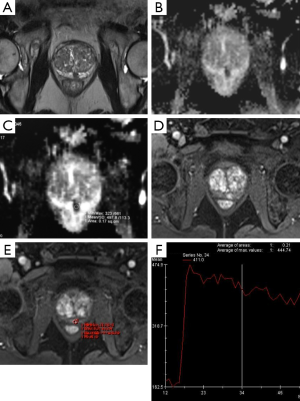
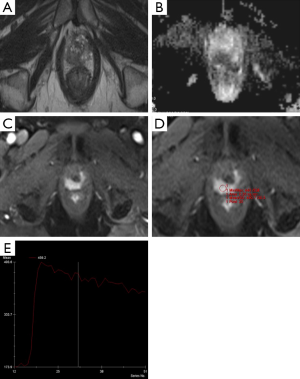
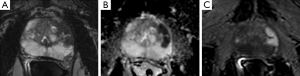

Full table
T2WI
T2WI, which reflects the water content of tissue, is the basis of mp-MRI. Because of the high resolution and sharp demarcation of the prostate capsule, T2WI can be used to determine prostate zonal anatomy and prostate cancer staging. In contrast to cancer in other organs, prostate cancer presents low signal intensity compared with adjacent glandular tissue because the abundant amount of water in the normal gland demonstrates high signal intensity on T2WI. This signal difference between normal and cancer tissue helps in cancer detection in the gland-rich peripheral zone. However, cancer identification on T2WI may be limited in the transitional zone, which does not contain a large amount of water. Moreover, the T2 shortening effect by biopsy-induced hemorrhage decreases signal intensity even in noncancerous tissue. Therefore, despite satisfactory performance as reported by early studies, recent literature has demonstrated the limitations of T2WI for prostate cancer detection. Therefore, the sensitivity and specificity of T2WI show significant variation in studies, 55-88% for sensitivity and 67-82% for specificity (15,16). Furthermore, such potential drawbacks of T2WI have introduced the need for mp-MRI.
DWI
Diffusion-weighted MRI is a functional imaging tool that measures the random Brownian motion of water molecules in tissue. The apparent diffusion coefficient (ADC) on MRI or the net displacement of molecules quantifies the restriction of water diffusion and is measured by acquiring at least two set of images with different magnetic field gradient durations and amplitudes (b value). Performing DWI requires at least two b factors for the calculation of ADC. Multipoint b value analyses increase the accuracy of the calculated ADC at the expense of increased scanning time and decrease in SNR. Earlier studies reported use of maximal b value of 1,000 s/mm2, but more recently it has been shown that a value of up to 2,000 s/mm2, which can be obtained on 3T scanners, may help to suppress signal from background normal prostate tissue and highlight the cancerous areas as hyperintense (17). Interpretation with high b values >1,000 s/mm2 is advocated for DWI in combination with ADC, with the hallmark of cancer being low ADC and iso to high signal on high b value DWI images (≥1,400 s/mm2). Limitations of DWI include increased noise and anatomic distortion of the image, especially at higher b values.
Studies have also shown an inverse correlation between quantitative ADC values and Gleason score, and may therefore help in assigning accurate risk stratification for selection of therapeutic options (18,19). But, there is significant overlap in confidence intervals that ADC cannot be used as a surrogate for Gleason score at this time, although most clinically significant cancers have a ADC value of <1,000 (20). DWI is a widely available technique and is considered to be the most important functional imaging sequence in mp-MRI. Functional imaging (DWI, DCE and MRSI), and in particular DWI, may help to differentiate cancer from benign abnormalities such as prostatitis, fibrosis, scar tissue, post-biopsy hemorrhage or post-irradiation in the peripheral zone. Therefore, DWI is considered as the dominant sequence for identifying tumors in the peripheral zone (21). It is also the most useful of all the functional imaging sequences for tumor detection in the transition zone. Multiple studies have shown DWI to be the most effective of the mp-MRI sequences for detecting prostate cancer, thereby improving the diagnostic performance of mp-MRI (16,22-25).
DCEI
DCEI is an imaging modality that is designed to evaluate the status of tumor angiogenesis. DCEI requires the acquisition of repeat gradient echo images before and after injection of contrast materials such as chelated gadolinium. Owing to rapid imaging, DCEI provides the time-intensity curve in each voxel. Because tumors are evidently associated with neoangiogenesis that induces an increase in the blood volume and transvascular permeability, tracing the dynamic flux of the contrast agent with DCEI shows strong and rapid contrast enhancement. Therefore, DCEI helps to monitor treatment effects as well as cancer detection. Recently, studies have also reported that DCEI can improve diagnostic performance for detecting local recurrence in patients who undergo radical prostatectomy (15,26). However, DCEI may cause false-positive diagnosis because inflammation is also accompanied by increased vascularity. Patient motion and peristalsis of the rectum during imaging may cause misregistration in imaging series, thereby disturbing the analysis of the time-intensity curve. The reported sensitivity and specificity of DCEI alone for prostate cancer detection also varies by reports (46-90% for sensitivity and 74-96% for specificity) (26).
MRSI
Among the sequences which comprise the mp-MRI, proton MRSI is the least frequently used and is mostly limited to the research setting. MRSI provides information about specific metabolites within prostatic tissue. The analysis is performed by measuring the resonance peaks of various biochemical metabolite levels such as citrate, creatine, and choline. Normal prostate tissue contains an abundant supply of zinc which inhibits aconitase and produces high levels of citrate. Citrate exhibits a unique peak on MR spectroscopy. On the other hand, in prostate cancer down regulation of the zinc transporters causes a decrease in zinc levels (27). This reduction in zinc decreases citrate levels by inducing oxidation. Choline levels correlate with cell turnover, as seen in prostate cancer. Thus, as cancers arise, citrate is expected to decline while choline is expected to rise. This ratio of choline to citrate is therefore an indicator of malignancy (Figure 4) (28-31). While MRSI is, in theory, a promising imaging sequence, it requires additional software expertise, training, and support and increases the overall mp-MRI scan time. In a multi-institutional study, it was determined that MR imaging alone was just as effective as MR imaging with MRSI and did not improve tumor localization in the peripheral zone, where most cancers occur. Also, out of the 110 patients in the final study group, only 50% were considered to have achieved good or excellent spectral quality notwithstanding the fact that the study was largely performed in excellent academic centers (32). For these reasons, MRSI has yet to become widely accepted in standard clinical practice; as a result, research has also slowed down with regard to MRSI. In a recent study of active surveillance in low-risk prostate cancer, it was determined that only T2W and DWI were independent predictors of biopsy upgrade (33). Spectroscopy was therefore not contributory. This study supports the argument against the routine use of MRSI in clinical practice and raises question about the future of MRSI as a component of mp-MRI.
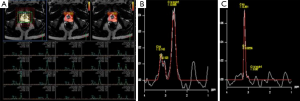
Role of mp-MRI in detection
Although the individual sequences are useful, T2WI in combination with two functional sequences has been shown to provide better characterization of tumor in the prostate (34,35). In a diagnostic meta-analysis of seven studies, de Rooij et al. revealed a high overall sensitivity and specificity on accuracy of mp-MRI using T2WI, DWI and DCEI. Pooled sensitivity and specificity were 0.74 and 0.88, respectively, with negative predictive value (NPV) ranging from 0.65 to 0.94 (36). In another study, mp-MRI showed good performance at detecting and ruling out clinically significant cancer, following at least one previous biopsy, with a NPV of 95% using transperineal template systemic biopsy as the gold standard (37). The authors concluded that mp-MRI can therefore be used as a triage test following a negative biopsy and thereby identify patients who can avoid further biopsies. A recently published study reported clinical NPV of mp-MRI at 89.6% for significant cancer over a longitudinal follow-up period of 5 years (38). Shakir et al. demonstrated that the benefit of MRI and targeted biopsy increases with increasing PSA levels and that the diagnostic usefulness and upgrading to clinically significant disease on biopsy occurred above a PSA threshold of 5.2 ng/mL (39).
While several studies have shown the benefit of functional imaging in detection of prostate cancer in the peripheral zone, functional imaging may have a limited role in evaluating cancers in the transition zone on mp-MRI because of the heterogenous appearance and enhancement secondary to benign prostatic hyperplasia (40). Hoeks et al. reported that DCE-MRI in particular did not show any additional benefit over T2WI for detection of cancer in the transition zone (41). In their study, accuracy of mp-MRI for detecting Gleason grades 4 and 5 in the transitional zone was 79% for T2WI and 72% when combined with DWI and DCEI. For low-risk disease, the accuracy levels were 66% for T2WI and 62% when combined with functional imaging. In another study, the authors reported that adding DWI to T2WI improved the accuracy of detecting prostate cancer in the transition zone (42).
Tumor volume is a documented prognostic factor for prostate cancer outcome, and is its correct estimation is mandatory for success of focal therapy, the new organ-sparing treatment technique that aims to selectively ablate locally confined, clinically significant index lesions, while sparing the rest of the prostate gland and the surrounding structures (43). Histologic architecture of the tumor affects quantitative MRI findings and is known to be a major predictor of tumor visibility on mp-MRI (44). Sparse or infiltrative tumor mixed with normal tissue may be present at the periphery of the MRI-visible “dense” tumor. Studies have shown that the greatest tumor volume on mp-MRI determined from images on any of the individual sequences provided a fairly accurate estimation of the tumor volume on whole-mount histology, although estimation was more accurate for larger tumors over 10 mm and >0.5 cc in volume than for small tumors (45-47).
Because prostate MRI interpretation can be subjective and inconsistent, suspicion scores for prostate cancer on MRI [Prostate Imaging and Reporting Archiving Data System (PI-RADS)] have been developed on a 1- to 5-point scale (based on fixed criteria) for improved standardization of MRI interpretation and reporting (13). The Likert scoring system is based on an overall impression of the reader and is a more subjective form of evaluation. Studies have shown higher interobserver reproducibility in the experienced readers than for less-experience readers for both the PI-RADS and the Likert scoring systems (48). A recent meta-analysis of 14 studies evaluating use of the PI-RADS scoring system for prostate cancer detection on mp-MRI showed good diagnostic accuracy (49). However, the PI-RADS scoring system is work in progress and PI-RADS version 2 has recently been published.
Role of mp-MRI in negative biopsy patients
In a meta-analysis including 14 studies and 698 patients, the mean cancer detection rate following a negative biopsy was 37.5% (range, 19.2-68.3%) (50). The pooled sensitivity and specificity by site analysis was 57% and 90%, respectively. The positive predictive value of mp-MRI in these studies ranged from 17 to 92. However, in many of these studies, biopsies were obtained by visual/cognitive assessment following mp-MRI. Hoeks et al. reported a cancer detection rate of 25% (108/438) in patients who had at least one previous negative biopsy for increased PSA and underwent subsequent mp-MRI and MRI guided in bore biopsy, with 87% of these cancers found to be clinically significant (51). The positive predictive value of mp-MRI in this study was 41% (108/265) by patient analysis and 33% (123/368) by site analysis. Similarly, Vourganti et al. reported a cancer detection rate of 37% (73/195) following a previous negative biopsy and suspicious mp-MRI (52). In their study, targeted biopsy using MRI-TRUS fusion upgraded in 28 patients and detected additional significant cancer in 12 patients, not detected by systematic biopsy. Recently, Sonn et al. also detected cancer in 34% (36/105) of patients using MRI-TRUS fusion following initial negative biopsy, with 72% of these being clinically significant. The positive predictive value of mp-MRI for highly suspicious lesions (PI-RAD scores of 4 and 5) was 50% (24/48 patients) (53).
Role of mp-MRI in active surveillance
Active surveillance is being utilized more frequently in the management of prostate cancer. The goal is to minimize the harm caused by overtreatment of low-risk disease while providing a means of identifying men with disease progression who require definitive treatment. A significant number of men on active surveillance protocols have a suspicious lesion that is identifiable on MRI (54). mp-MRI may prove to be particularly useful in this setting because suspicious lesions can be targeted with fusion biopsy leading to preferential sampling of prostate cancer tissue. This means that prostate cancer progression can be detected more efficiently and accurately. Growing evidence supports the role of repeat mp-MRI of the prostate and fusion biopsy to improve monitoring of men on active surveillance. In a retrospective analysis, Abdi et al. (n=603) demonstrated that mp-MRI of the prostate with the option of subsequent fusion biopsy improves the detection of prostate cancer progression for men under active surveillance (55). Walton Diaz et al. (n=152) demonstrated that stable mp-MRI findings were associated with Gleason score stability on biopsy (56). Importantly, only 2.9 fusion biopsies were needed to detect one case of Gleason progression compared with 8.74 saturation biopsies. According to the authors, mp-MRI may be a promising means of reducing the number of biopsies for men on active surveillance.
Siddiqui et al. (n=85) found that mp-MRI could reduce the number of repeat biopsies by up to 68% for men on active surveillance (57). A tumor that is not detected on mp-MRI is more likely to be low risk, and according to Johnson et al., the risk of biologically significant disease in patients with a negative mp-MRI result is low enough to justify deferring definitive treatment without biopsy (58). The findings in these studies are promising and certainly warrant evaluation in large prospective trials. The Prostate Cancer Research International: Active Surveillance (PRIAS) study, which is the largest prospective study evaluating active surveillance, has commenced recruiting eligible patients to have mp-MRI incorporated into the surveillance data. This will provide reliable information with regards to the feasibility of mp-MRI in the context of active surveillance (59).
Role of mp-MRI in detection of recurrence after radical prostatectomy
Most post-prostatectomy recurrent prostate cancer is diagnosed by PSA elevation. Once PSA increment is detected, it is essential to identify whether prostate cancer has recurred at a local or a distant site to determine the treatment modalities. In current practice, imaging or pathological evidence of local recurrence is not necessary to initiate local salvage treatment because current imaging techniques cannot adequately detect small-sized local recurrence.
In a recent meta-analysis, mp-MRI was reported to have sufficient accuracy for detecting local recurrence in patients with low PSA and small-sized recurrence (60). Recently, an increasing number of studies have been published reporting the acceptable diagnostic accuracy of mp-MRI for detecting local recurrence. Among the functional sequences, DCEI has been regarded as the most reliable sequence in detecting local recurrence after prostatectomy (61,62). However, it must be taken into account that vascularity and contrast enhancement can be reduced in patients who have received androgen deprivation therapy. In this regard, the accuracy of DCEI might be reduced in patients who undergo androgen deprivation therapy. Sensitivity and specificity of DCEI alone for detecting local recurrence after radical prostatectomy range from 88% to 100% and from 45% to 97%, respectively (63-66). Moreover, DCEI increased interobserver agreement and addition of DCEI to T2WI significantly increased accuracy for detecting local recurrence (63).
Recently, some studies have shown that DWI is also a reliable sequence in detecting local recurrence (64,65). Moreover, the combination of DWI and DCEI seemed to have more consistent specificity of 82% to 87% compared with DCEI alone (65,66). Accuracy of combined functional sequences has not been sufficiently reported (67,68). According to a recent study, T2WI plus DCEI has the highest sensitivity of 97% followed by DCEI alone and T2WI plus DWI plus DCEI (62).
Conclusions
The current literature indicates that mp-MRI of the prostate is a promising technology within prostate cancer management. Robust data to confirm many of these findings are still needed. Despite promising data indicating that Gleason score can be predicted without a tissue sample (particularly with DWI), such findings should be interpreted cautiously in the clinical setting, particularly in the scenario of an elevated PSA test and negative mp-MRI of the prostate. The clinical confidence in this aspect of the technology is justifiably more guarded compared with the academic excitement. The largest benefit may come from reduction of unnecessary biopsies which could in turn prevent overdiagnosis and overtreatment. It also has the potential to decrease the number of missed clinically significant cancers. Like any new technology, it should be treated judiciously and used in combination with current clinical tools for risk stratification. More likely than not, the gold standard for evaluating mp-MRI is direct comparison of radiology to histopathology. The development of a more sophisticated, standardized model for correlating radiological parameters with histopathology in addition to higher volumes of good quality data is the logical next research pathway.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [PubMed]
- Chou R, Croswell JM, Dana T, Bougatsos C, Blazina I, Fu R, Gleitsmann K, Koenig HC, Lam C, Maltz A, Rugge JB, Lin K. Screening for prostate cancer: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2011;155:762-71. [PubMed]
- Panebianco V, Barchetti F, Sciarra A, Ciardi A, Indino EL, Papalia R, Gallucci M, Tombolini V, Gentile V, Catalano C. Multiparametric magnetic resonance imaging vs. standard care in men being evaluated for prostate cancer: a randomized study. Urol Oncol 2015;33:17.e1-7.
- Schimmöller L, Quentin M, Arsov C, Hiester A, Buchbender C, Rabenalt R, Albers P, Antoch G, Blondin D. MR-sequences for prostate cancer diagnostics: validation based on the PI-RADS scoring system and targeted MR-guided in-bore biopsy. Eur Radiol 2014;24:2582-9. [PubMed]
- Petrillo A, Fusco R, Setola SV, Ronza FM, Granata V, Petrillo M, Carone G, Sansone M, Franco R, Fulciniti F, Perdonà S. Multiparametric MRI for prostate cancer detection: performance in patients with prostate-specific antigen values between 2.5 and 10 ng/mL. J Magn Reson Imaging 2014;39:1206-12. [PubMed]
- Manenti G, Nezzo M, Chegai F, Vasili E, Bonanno E, Simonetti G. DWI of Prostate Cancer: Optimal b-Value in Clinical Practice. Prostate Cancer 2014;2014:868269.
- Marcus DM, Rossi PJ, Nour SG, Jani AB. The impact of multiparametric pelvic magnetic resonance imaging on risk stratification in patients with localized prostate cancer. Urology 2014;84:132-7. [PubMed]
- Lawrence EM, Gallagher FA, Barrett T, Warren AY, Priest AN, Goldman DA, Sala E, Gnanapragasam VJ. Preoperative 3-T diffusion-weighted MRI for the qualitative and quantitative assessment of extracapsular extension in patients with intermediate- or high-risk prostate cancer. AJR Am J Roentgenol 2014;203:W280-6. [PubMed]
- Jie C, Rongbo L, Ping T. The value of diffusion-weighted imaging in the detection of prostate cancer: a meta-analysis. Eur Radiol 2014;24:1929-41. [PubMed]
- Choi H, Underwood M, Boonsirikamchai P, Matin S, Troncoso P, Ma J. Technical challenges in 3 T magnetic resonance spectroscopic imaging of the prostate-A single-institution experience. Quant Imaging Med Surg 2014;4:251-8. [PubMed]
- Esen M, Onur MR, Akpolat N, Orhan I, Kocakoc E. Utility of ADC measurement on diffusion-weighted MRI in differentiation of prostate cancer, normal prostate and prostatitis. Quant Imaging Med Surg 2013;3:210-6. [PubMed]
- Li L, Wang L, Feng Z, Hu Z, Wang G, Yuan X, Wang H, Hu D. Prostate cancer magnetic resonance imaging (MRI): multidisciplinary standpoint. Quant Imaging Med Surg 2013;3:100-12. [PubMed]
- Barentsz JO, Richenberg J, Clements R, Choyke P, Verma S, Villeirs G, Rouviere O, Logager V, Fütterer JJ; European Society of Urogenital Radiology. ESUR prostate MR guidelines 2012. Eur Radiol 2012;22:746-57. [PubMed]
- Shah ZK, Elias SN, Abaza R, Zynger DL, DeRenne LA, Knopp MV, Guo B, Schurr R, Heymsfield SB, Jia G. Performance comparison of 1.5-T endorectal coil MRI with 3.0-T nonendorectal coil MRI in patients with prostate cancer. Acad Radiol 2015;22:467-74. [PubMed]
- Yoo S, Kim JK, Jeong IG. Multiparametric magnetic resonance imaging for prostate cancer: A review and update for urologists. Korean J Urol 2015;56:487-97. [PubMed]
- Wu LM, Xu JR, Ye YQ, Lu Q, Hu JN. The clinical value of diffusion-weighted imaging in combination with T2-weighted imaging in diagnosing prostate carcinoma: a systematic review and meta-analysis. AJR Am J Roentgenol 2012;199:103-10. [PubMed]
- Kitajima K, Takahashi S, Ueno Y, Yoshikawa T, Ohno Y, Obara M. Clinical utility of apparent diffusion coefficient values obtained using high b-value when diagnosing prostate cancer using 3 tesla MRI: Comparison between ultra-high b-value (2000 s/mm2) and standard high b-value (1000 s/mm2). J Magn Reson Imaging 2012;36:198-205. [PubMed]
- Hambrock T, Somford DM, Huisman HJ, van Oort IM, Witjes JA, Hulsbergen-van de Kaa CA, Scheenen T, Barentsz JO. Relationship between apparent diffusion coefficients at 3.0-T MR imaging and Gleason grade in peripheral zone prostate cancer. Radiology 2011;259:453-61. [PubMed]
- Turkbey B, Shah VP, Pang Y, Bernardo M, Xu S, Kruecker J, Locklin J, Baccala AA Jr, Rastinehad AR, Merino MJ, Shih JH, Wood BJ, Pinto PA, Choyke PL. Is apparent diffusion coefficient associated with clinical risk scores for prostate cancers that are visible on 3-T MR images? Radiology 2011;258:488-95. [PubMed]
- Kumar V, Jagannathan NR, Kumar R, Thulkar S, Gupta SD, Dwivedi SN, Hemal AK, Gupta NP. Apparent diffusion coefficient of the prostate in men prior to biopsy: determination of a cut-off value to predict malignancy of the peripheral zone. NMR Biomed 2007;20:505-11. [PubMed]
- Haider MA, van der Kwast TH, Tanguay J, Evans AJ, Hashmi AT, Lockwood G, Trachtenberg J. Combined T2-weighted and diffusion-weighted MRI for localization of prostate cancer. AJR Am J Roentgenol 2007;189:323-8. [PubMed]
- Tan CH, Wei W, Johnson V, Kundra V. Diffusion-weighted MRI in the detection of prostate cancer: meta-analysis. AJR Am J Roentgenol 2012;199:822-9. [PubMed]
- Wu LM, Xu JR, Gu HY, Hua J, Chen J, Zhang W, Zhu J, Ye YQ, Hu J. Usefulness of diffusion-weighted magnetic resonance imaging in the diagnosis of prostate cancer. Acad Radiol 2012;19:1215-24. [PubMed]
- Katahira K, Takahara T, Kwee TC, Oda S, Suzuki Y, Morishita S, Kitani K, Hamada Y, Kitaoka M, Yamashita Y. Ultra-high-b-value diffusion-weighted MR imaging for the detection of prostate cancer: evaluation in 201 cases with histopathological correlation. Eur Radiol 2011;21:188-96. [PubMed]
- Ghai S, Haider MA. Multiparametric-MRI in diagnosis of prostate cancer. Indian J Urol 2015;31:194-201. [PubMed]
- Verma S, Turkbey B, Muradyan N, Rajesh A, Cornud F, Haider MA, Choyke PL, Harisinghani M. Overview of dynamic contrast-enhanced MRI in prostate cancer diagnosis and management. AJR Am J Roentgenol 2012;198:1277-88. [PubMed]
- Ogura K, Maekawa S, Okubo K, Aoki Y, Okada T, Oda K, Watanabe Y, Tsukayama C, Arai Y. Dynamic endorectal magnetic resonance imaging for local staging and detection of neurovascular bundle involvement of prostate cancer: correlation with histopathologic results. Urology 2001;57:721-6. [PubMed]
- Mazaheri Y, Shukla-Dave A, Hricak H, Fine SW, Zhang J, Inurrigarro G, Moskowitz CS, Ishill NM, Reuter VE, Touijer K, Zakian KL, Koutcher JA. Prostate cancer: identification with combined diffusion-weighted MR imaging and 3D 1H MR spectroscopic imaging--correlation with pathologic findings. Radiology 2008;246:480-8. [PubMed]
- Jung JA, Coakley FV, Vigneron DB, Swanson MG, Qayyum A, Weinberg V, Jones KD, Carroll PR, Kurhanewicz J. Prostate depiction at endorectal MR spectroscopic imaging: investigation of a standardized evaluation system. Radiology 2004;233:701-8. [PubMed]
- Scheenen TW, Heijmink SW, Roell SA, Hulsbergen-Van de Kaa CA, Knipscheer BC, Witjes JA, Barentsz JO, Heerschap A. Three-dimensional proton MR spectroscopy of human prostate at 3 T without endorectal coil: feasibility. Radiology 2007;245:507-16. [PubMed]
- Walker P, Provent P, Tizon X, Crehange G, Duchamp O, Brunotte F. In-vivo metabolic characterization of healthy prostate and orthotopic prostate cancer in rats using proton magnetic resonance spectroscopy at 4.7 T. Acta Radiol 2013;54:121-6. [PubMed]
- Kobus T, Hambrock T, Hulsbergen-van de Kaa CA, Wright AJ, Barentsz JO, Heerschap A, Scheenen TW. In vivo assessment of prostate cancer aggressiveness using magnetic resonance spectroscopic imaging at 3 T with an endorectal coil. Eur Urol 2011;60:1074-80. [PubMed]
- Weinreb JC, Blume JD, Coakley FV, Wheeler TM, Cormack JB, Sotto CK, Cho H, Kawashima A, Tempany-Afdhal CM, Macura KJ, Rosen M, Gerst SR, Kurhanewicz J. Prostate cancer: sextant localization at MR imaging and MR spectroscopic imaging before prostatectomy--results of ACRIN prospective multi-institutional clinicopathologic study. Radiology 2009;251:122-33. [PubMed]
- Fütterer JJ, Heijmink SW, Scheenen TW, Veltman J, Huisman HJ, Vos P, Hulsbergen-Van de Kaa CA, Witjes JA, Krabbe PF, Heerschap A, Barentsz JO. Prostate cancer localization with dynamic contrast-enhanced MR imaging and proton MR spectroscopic imaging. Radiology 2006;241:449-58. [PubMed]
- Tanimoto A, Nakashima J, Kohno H, Shinmoto H, Kuribayashi S. Prostate cancer screening: the clinical value of diffusion-weighted imaging and dynamic MR imaging in combination with T2-weighted imaging. J Magn Reson Imaging 2007;25:146-52. [PubMed]
- de Rooij M, Hamoen EH, Fütterer JJ, Barentsz JO, Rovers MM. Accuracy of multiparametric MRI for prostate cancer detection: a meta-analysis. AJR Am J Roentgenol 2014;202:343-51. [PubMed]
- Abd-Alazeez M, Ahmed HU, Arya M, Charman SC, Anastasiadis E, Freeman A, Emberton M, Kirkham A. The accuracy of multiparametric MRI in men with negative biopsy and elevated PSA level--can it rule out clinically significant prostate cancer? Urol Oncol 2014;32:45.e17-22.
- Itatani R, Namimoto T, Atsuji S, Katahira K, Morishita S, Kitani K, Hamada Y, Kitaoka M, Nakaura T, Yamashita Y. Negative predictive value of multiparametric MRI for prostate cancer detection: outcome of 5-year follow-up in men with negative findings on initial MRI studies. Eur J Radiol 2014;83:1740-5. [PubMed]
- Shakir NA, George AK, Siddiqui MM, Rothwax JT, Rais-Bahrami S, Stamatakis L, Su D, Okoro C, Raskolnikov D, Walton-Diaz A, Simon R, Turkbey B, Choyke PL, Merino MJ, Wood BJ, Pinto PA. Identification of threshold prostate specific antigen levels to optimize the detection of clinically significant prostate cancer by magnetic resonance imaging/ultrasound fusion guided biopsy. J Urol 2014;192:1642-8. [PubMed]
- Delongchamps NB, Rouanne M, Flam T, Beuvon F, Liberatore M, Zerbib M, Cornud F. Multiparametric magnetic resonance imaging for the detection and localization of prostate cancer: combination of T2-weighted, dynamic contrast-enhanced and diffusion-weighted imaging. BJU Int 2011;107:1411-8. [PubMed]
- Hoeks CM, Hambrock T, Yakar D, Hulsbergen-van de Kaa CA, Feuth T, Witjes JA, Fütterer JJ, Barentsz JO. Transition zone prostate cancer: detection and localization with 3-T multiparametric MR imaging. Radiology 2013;266:207-17. [PubMed]
- Jung SI, Donati OF, Vargas HA, Goldman D, Hricak H, Akin O. Transition zone prostate cancer: incremental value of diffusion-weighted endorectal MR imaging in tumor detection and assessment of aggressiveness. Radiology 2013;269:493-503. [PubMed]
- Cornud F, Khoury G, Bouazza N, Beuvon F, Peyromaure M, Flam T, Zerbib M, Legmann P, Delongchamps NB. Tumor target volume for focal therapy of prostate cancer-does multiparametric magnetic resonance imaging allow for a reliable estimation? J Urol 2014;191:1272-9. [PubMed]
- Rosenkrantz AB, Mendrinos S, Babb JS, Taneja SS. Prostate cancer foci detected on multiparametric magnetic resonance imaging are histologically distinct from those not detected. J Urol 2012;187:2032-8. [PubMed]
- Bratan F, Melodelima C, Souchon R, Hoang Dinh A, Mège-Lechevallier F, Crouzet S, Colombel M, Gelet A, Rouvière O. How accurate is multiparametric MR imaging in evaluation of prostate cancer volume? Radiology 2015;275:144-54. [PubMed]
- Nakashima J, Tanimoto A, Imai Y, Mukai M, Horiguchi Y, Nakagawa K, Oya M, Ohigashi T, Marumo K, Murai M. Endorectal MRI for prediction of tumor site, tumor size, and local extension of prostate cancer. Urology 2004;64:101-5. [PubMed]
- Coakley FV, Kurhanewicz J, Lu Y, Jones KD, Swanson MG, Chang SD, Carroll PR, Hricak H. Prostate cancer tumor volume: measurement with endorectal MR and MR spectroscopic imaging. Radiology 2002;223:91-7. [PubMed]
- Rosenkrantz AB, Lim RP, Haghighi M, Somberg MB, Babb JS, Taneja SS. Comparison of interreader reproducibility of the prostate imaging reporting and data system and likert scales for evaluation of multiparametric prostate MRI. AJR Am J Roentgenol 2013;201:W612-8. [PubMed]
- Hamoen EH, de Rooij M, Witjes JA, Barentsz JO, Rovers MM. Use of the Prostate Imaging Reporting and Data System (PI-RADS) for Prostate Cancer Detection with Multiparametric Magnetic Resonance Imaging: A Diagnostic Meta-analysis. Eur Urol 2015;67:1112-21. [PubMed]
- Zhang ZX, Yang J, Zhang CZ, Li KA, Quan QM, Wang XF, Wang H, Zhang GX. The value of magnetic resonance imaging in the detection of prostate cancer in patients with previous negative biopsies and elevated prostate-specific antigen levels: a meta-analysis. Acad Radiol 2014;21:578-89. [PubMed]
- Hoeks CM, Schouten MG, Bomers JG, Hoogendoorn SP, Hulsbergen-van de Kaa CA, Hambrock T, Vergunst H, Sedelaar JP, Fütterer JJ, Barentsz JO. Three-Tesla magnetic resonance-guided prostate biopsy in men with increased prostate-specific antigen and repeated, negative, random, systematic, transrectal ultrasound biopsies: detection of clinically significant prostate cancers. Eur Urol 2012;62:902-9. [PubMed]
- Vourganti S, Rastinehad A, Yerram NK, Nix J, Volkin D, Hoang A, Turkbey B, Gupta GN, Kruecker J, Linehan WM, Choyke PL, Wood BJ, Pinto PA. Multiparametric magnetic resonance imaging and ultrasound fusion biopsy detect prostate cancer in patients with prior negative transrectal ultrasound biopsies. J Urol 2012;188:2152-7. [PubMed]
- Sonn GA, Chang E, Natarajan S, Margolis DJ, Macairan M, Lieu P, Huang J, Dorey FJ, Reiter RE, Marks LS. Value of targeted prostate biopsy using magnetic resonance-ultrasound fusion in men with prior negative biopsy and elevated prostate-specific antigen. Eur Urol 2014;65:809-15. [PubMed]
- Schoots IG, Petrides N, Giganti F, Bokhorst LP, Rannikko A, Klotz L, Villers A, Hugosson J, Moore CM. Magnetic resonance imaging in active surveillance of prostate cancer: a systematic review. Eur Urol 2015;67:627-36. [PubMed]
- Abdi H, Pourmalek F, Zargar H, Walshe T, Harris AC, Chang SD, Eddy C, So AI, Gleave ME, Machan L, Goldenberg SL, Black PC. Multiparametric magnetic resonance imaging enhances detection of significant tumor in patients on active surveillance for prostate cancer. Urology 2015;85:423-8. [PubMed]
- Walton Diaz A, Shakir NA, George AK, Rais-Bahrami S, Turkbey B, Rothwax JT, Stamatakis L, Hong CW, Siddiqui MM, Okoro C, Raskolnikov D, Su D, Shih J, Han H, Parnes HL, Merino MJ, Simon RM, Wood BJ, Choyke PL, Pinto PA. Use of serial multiparametric magnetic resonance imaging in the management of patients with prostate cancer on active surveillance. Urol Oncol 2015;33:202.e1-7.
- Siddiqui MM, Truong H, Rais-Bahrami S, Stamatakis L, Logan J, Walton-Diaz A, Turkbey B, Choyke PL, Wood BJ, Simon RM, Pinto PA. Clinical implications of a multiparametric magnetic resonance imaging based nomogram applied to prostate cancer active surveillance. J Urol 2015;193:1943-9. [PubMed]
- Johnson LM, Choyke PL, Figg WD, Turkbey B. The role of MRI in prostate cancer active surveillance. Biomed Res Int 2014;2014:203906.
- Bul M, Zhu X, Valdagni R, Pickles T, Kakehi Y, Rannikko A, Bjartell A, van der Schoot DK, Cornel EB, Conti GN, Boevé ER, Staerman F, Vis-Maters JJ, Vergunst H, Jaspars JJ, Strölin P, van Muilekom E, Schröder FH, Bangma CH, Roobol MJ. Active surveillance for low-risk prostate cancer worldwide: the PRIAS study. Eur Urol 2013;63:597-603. [PubMed]
- Alfarone A, Panebianco V, Schillaci O, Salciccia S, Cattarino S, Mariotti G, Gentilucci A, Von Heland M, Passariello R, Gentile V, Sciarra A. Comparative analysis of multiparametric magnetic resonance and PET-CT in the management of local recurrence after radical prostatectomy for prostate cancer. Crit Rev Oncol Hematol 2012;84:109-21. [PubMed]
- Linder BJ, Kawashima A, Woodrum DA, Tollefson MK, Karnes J, Davis BJ, Rangel LJ, King BF, Mynderse LA. Early localization of recurrent prostate cancer after prostatectomy by endorectal coil magnetic resonance imaging. Can J Urol 2014;21:7283-9. [PubMed]
- Roy C, Foudi F, Charton J, Jung M, Lang H, Saussine C, Jacqmin D. Comparative sensitivities of functional MRI sequences in detection of local recurrence of prostate carcinoma after radical prostatectomy or external-beam radiotherapy. AJR Am J Roentgenol 2013;200:W361-8. [PubMed]
- Wassberg C, Akin O, Vargas HA, Shukla-Dave A, Zhang J, Hricak H. The incremental value of contrast-enhanced MRI in the detection of biopsy-proven local recurrence of prostate cancer after radical prostatectomy: effect of reader experience. AJR Am J Roentgenol 2012;199:360-6. [PubMed]
- Panebianco V, Barchetti F, Sciarra A, Musio D, Forte V, Gentile V, Tombolini V, Catalano C. Prostate cancer recurrence after radical prostatectomy: the role of 3-T diffusion imaging in multi-parametric magnetic resonance imaging. Eur Radiol 2013;23:1745-52. [PubMed]
- Cha D, Kim CK, Park SY, Park JJ, Park BK. Evaluation of suspected soft tissue lesion in the prostate bed after radical prostatectomy using 3T multiparametric magnetic resonance imaging. Magn Reson Imaging 2015;33:407-12. [PubMed]
- Kitajima K, Murphy RC, Nathan MA, Froemming AT, Hagen CE, Takahashi N, Kawashima A. Detection of recurrent prostate cancer after radical prostatectomy: comparison of 11C-choline PET/CT with pelvic multiparametric MR imaging with endorectal coil. J Nucl Med 2014;55:223-32. [PubMed]
- Hsu CC, Hsu H, Pickett B, Crehange G, Hsu IC, Dea R, Weinberg V, Gottschalk AR, Kurhanewicz J, Shinohara K, Roach M 3rd. Feasibility of MR imaging/MR spectroscopy-planned focal partial salvage permanent prostate implant (PPI) for localized recurrence after initial PPI for prostate cancer. Int J Radiat Oncol Biol Phys 2013;85:370-7. [PubMed]
- Créhange G, Parfait S, Liegard M, Maingon P, Ben Salem D, Cochet A, Funes de la Vega M, Cormier L, Bonnetain F, Mirjolet C, Brunotte F, Walker PM. Tumor volume and metabolism of prostate cancer determined by proton magnetic resonance spectroscopic imaging at 3T without endorectal coil reveal potential clinical implications in the context of radiation oncology. Int J Radiat Oncol Biol Phys 2011;80:1087-94. [PubMed]


