Preoperative portal vein embolization in liver cancer: indications, techniques and outcomes
Introduction
Complete resection of hepatic tumors remains the first choice for curative treatment of primary and secondary liver malignancies, giving the patient the only chance of long-term survival. In up to 45% of primary and secondary liver tumors, extended liver resection is necessary to achieve clear resection margins (1). The reason for unresectability is that often the remnant liver is of insufficient volume to support postoperative liver function, which itself is still the principal cause of postoperative death after major hepatectomy. The mortality rate after major liver resection ranges from 3.2% to 7% in patients with non-injured liver parenchyma and increases up to 32% in patients with cirrhosis (1-3). It has been demonstrated that liver failure is directly related to the size of remnant functional liver volume, and various procedures have been developed to induce liver regeneration. Preoperative embolization of the portal vein (PV) branches feeding the hepatic segments to be resected reduced the risk of postoperative liver failure after major liver resection and increased the number of resectable patients (2-5). In this article, we discuss and illustrate normal PV anatomy and variants, indications and contraindications for portal vein embolization (PVE), technical considerations and periprocedural issues related to percutaneous transhepatic PVE, and potential complications of the procedure.
Anatomy
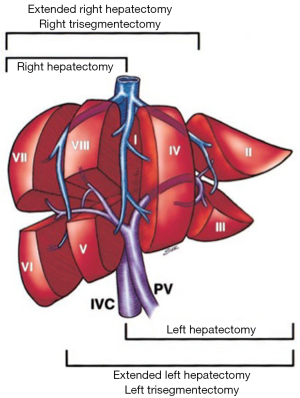
A comprehensive knowledge of functional liver anatomy is imperative for performing PVE. The most widely used classification system was proposed in 1957 by Couinaud (6). The liver is divided into two hemilivers (left and right, separated by the main portal fissure) and eight segments. Hepatic segmentation is based on the distribution of the portal pedicles and the location of the hepatic veins.
Normal PV anatomy
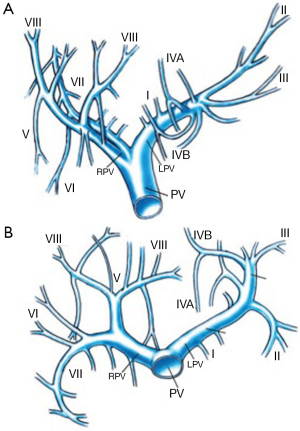
The PV is formed in the retroperitoneum by the confluence of the superior mesenteric vein and the splenic vein behind the neck of the pancreas and courses behind the duodenal bulb. The main PV and the right and left portal veins (LPVs) are in the hilar fissure. The portal bifurcation may be extrahepatic (48% of cases), intrahepatic (26%), or located right at the entrance of the liver (26%) (7,8). Figures 1 and 2 illustrate the most common portal venous anatomy. On the right, there are usually two sectoral portal branches (anterior and posterior); on the left, there are two parts to the (main) LPV: the extrahepatic portion [the horizontal part (hp)] and the intrahepatic portion (the umbilical vertical part). In general, the sectoral branch divides into several segmental portal branches, which in turn supply the various segments. One segmental branch usually supplies segments II, VI, and VII and, more rarely, segment III. Segments IV, V, and VIII are commonly supplied by more than one segmental branch. Segmental veins then divide into subsegmental branches, which further divide into small veins leading to the portal venule of the liver acinus (9).
PV variants
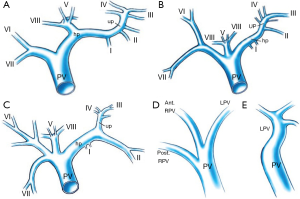
Anatomic variants of the PV are uncommon (10-15% of cases) (Figure 3) (10). However, when present, they are important to recognize because they may have profound implications for whether PVE or subsequent resection can be performed successfully. In a small portion (11%) of the population, the PV divides into one left and two right portal branches. This variant, known as portal trifurcation, is present if three branches stem from the main portal trunk: the posterior branch, the anterior branch, and the left main branch (Figure 3B). In addition, the right anterior segment PV may branch from the left main PV (4% of cases), or the left main PV may branch from the right anterior PV. Alternatively, the right posterior branch may stem from the main portal trunk, with the anterior branch forming a bifurcation with the LPV (5% of cases). Quadrifurcation of the PV can also occur, consisting of a branch for segment VII, a branch for segment VI, an anterior branch, and a left main portal branch (LPV) (Figure 3C). In exceptional cases, a branch for subsegment IVb or an additional branch for segments VI, VII, or even VIII may stem from the portal bifurcation. Only very rarely (1% of cases) are bifurcation of the PV completely absent [no right portal vein (RPV)] (Figure 3E) (11). When this occurs, the solitary PV in the hilum passes through the entire liver, either from right to left or from left to right. Failure to recognize this variation in the setting of hilar portal ligation leads to hepatic failure and death. Resection or liver transplantation may require PV resection and reconstruction, which greatly increases the complexity of these procedures (11). Additional variations can occur in both the right and left portal systems. It is extremely important to be aware of portal anomalies because failure to do so can lead to non-target embolization with potential risk to the future liver remnants (FLR).
Indications
At present, four factors are important to consider when deciding whether to perform PVE. First, the ratio of FLR to total estimated liver volume (TELV) should be calculated. Second, cases need to be categorized into those with and those without underlying liver disease because this factor will determine how much FLR is needed to reduce postoperative morbidity and mortality. The minimum absolute liver volume necessary to support postresection hepatic function has not been clearly defined. However, a FLR/TELV ratio of at least 25% is recommended in patients with otherwise normal livers, with a ratio of at least 40% in patients in whom the liver is considered compromised (from chronic liver disease or high-dose chemotherapy) (12-17). When FLR/TELV ratios are below these levels, PVE may be performed in an attempt to increase FLR volume. Third, the presence of systemic disease such as diabetes mellitus may limit hepatic hypertrophy. Insulin is a comitogenic factor with HGF that often leads to slower rates of regeneration (18). Fourth, planning for the type and extent of the anticipated surgical procedure (right hepatectomy and pancreaticoduodenectomy) is important because more functional hepatic reserve may be required to reduce postoperative morbidity.
Contraindications
Patients with metastatic diseases such as distant metastases or periportal lymphadenopathy cannot undergo resection and therefore are not candidates for PVE. Patients with bilobar multiple metastases were not considered as the candidates for PVE before (9), but recent studies confirm that some of these patients can benefit from PVE in combination with two-stage hepatectomy (19). Other relative contraindications for PVE include an uncorrectable coagulopathy, tumor invasion of the PV, tumor precluding safe transhepatic access, biliary dilatation (in cases of biliary tree obstruction, drainage is recommended), portal hypertension, and renal failure that requires dialysis. PVE in cases of tumor invasion of the PV may not be warranted because there may be no significant benefit from the procedure (5).
Techniques
Pre-embolization work-up
Prior to PVE, a complete patient history is taken and a thorough physical examination performed. Laboratory studies including complete blood cell count, prothrombin time, liver function tests, and blood urea nitrogen/creatinine levels are essential prior to PVE. If patient has an elevated total bilirubin (>3.0 mg/dL), percutaneous or endoscopic biliary drainage is beneficial. CT or MRI scanning is a fundamental radiological investigation prior to PVE, for it documents the extent of disease (extrahepatic disease or involvement of the planned FLR), FLR size, and portal venous anatomy (5,9).
Patients should be informed that this procedure is not an antitumoral treatment but a treatment made to increase safety or to enable a surgical procedure. Minor complications are encountered in 20% to 25% of cases and are mainly associated with slight fever and abdominal discomfort and pain. Major complications are infrequent and mainly include infection and subcapsular hematoma, hemobilia, and PV thrombosis (<2% of cases). Mortality due to PVE has not been reported (19,20).
PVE technique
Although general anesthetic may be requested, the procedure is most often performed with local anesthetic (1% lidocaine hydrochloride) and intravenously administered sedatives that allow the patient to remain conscious. Access to the portal system should be done under ultrasound guidance to puncture a peripheral branch (21). Access can be obtained by way of controlateral approach (puncture of the left portal branch and embolization of the right portal branches) or ipsilateral approach (puncture of the right portal branch to embolize right portal branches). The advantage of the controlateral approach is easier catheterization, but there is a risk of damage to the FLR. Five-French materials (catheter or introductory sheath) are usually recommended. The catheter should be placed at the splenomesenteric confluence to perform a portography to visualize portal anatomy, including its variations, and to localize segment IV branches. Measurement of portal pressure is not routinely performed in patients with normal liver. In cirrhotic patients, measuring the portal and central venous pressures is useful to determine whether the patient has a portosystemic gradient >12 mmHg in which case the patient is at major risk of perisurgical complications (20,22,23). These patients are not eligible for PVE. The aim of embolization is complete obstruction of the targeted branches and redistribution of flow to the FLR branches only. Final portography is mandatory to verify this objective. A final pressure measurement should be obtained at the end of the procedure in patients with chronic liver disease to document portal pressure increase, which is usually approximately 3 mmHg. Embolization of segment IV branches is recommended in patients with tumors who are undergoing extended right hepatectomy. However, if embolization of that segment causes risk of reflux into the portal branch of the FRL, such embolization must not be performed because any major reflux into FRL portal branches might preclude surgery.
Choice of embolic agent
Various embolic materials have been used. Some products are not recommended due to reported recanalization or lower induced hypertrophy (Table 1). Gelfoam is associated with a high rate of PV recanalization and seems less efficient than other products (21,24-27). Nonspherical polyvinyl alcohol (PVA) particles have been used but are less efficient than spherical particles (36). Direct intraportal alcohol injection has been described. Although efficient, it is hard to control and has been associated with significant morbidity (liver necrosis, PV thrombosis) (14).

Full table
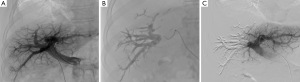
Recommended products (36-38) include the following. Mixture of n-butyl-cyanoacrylate and iodized oil has been described extensively as showing good results and low morbidity. Usually a mixture of one part n-butyl 2-cyanoacrylate (NBCA) (Histoacryl®, B/Braun, Germany; or Glubran®2, GEM, Italy) to one or two parts Lipiodol (Guerbet, France) is used. Injections of small aliquots in between abundant flushing with nonionic liquid, such as dextran or glucose 5%, is the most commonly reported technique (31-33). In our practice, we used a higher dilution mixture (1:8 ratio) to obtain a very distal embolization (39). Glue allows for fast procedure in comparison with other embolic agents (Figure 4). Spherical microparticles are associated with coil embolization, which is mostly described in North American reports, and have been reported to be superior to nonspherical PVA (28-30). It seems as efficient as NBCA, although it has never been compared in randomized trials. Most teams start with 300- to 500-µm particles and finish with 700- to 900-µm particles (20). Coils are used at the end of the procedure to allow for complete occlusion of the proximal trunk. It is advisable to avoid all too proximal occlusions and rather leave 1 cm unembolized segment of the right portal branch to facilitate surgical ligation at the time of liver resection. Association of fibrin glue with iodized oil has mostly been described in Japan and has the drawback of requiring special catheters that are only available in Asia (34,35). Amplatzer vascular plugs can be used instead of coils for occlusion of the proximal trunk or before glue embolization by ipsilateral approach to avoid reflux in the controlateral branches (30).
Post-procedural monitoring
Evaluation for signs of postembolization syndrome or liver insufficiency includes review of patient symptoms, clinical signs, and laboratory data (such as elevated white blood cell count, increasing transaminase levels, or prothrombin time). Patients are discharged when they are clinically stable and without complaints, usually the next day. Repeat CT is performed after 2-4 weeks to assess FLR hypertrophy and disease spread. If liver regeneration occurs and there is no spread of disease that would contraindicate the procedure, resection is performed. Otherwise, follow-up CT is performed at monthly intervals. Because the minimum safe FLR volume that would contraindicate resection has not yet been determined, we still perform resection in all patients who demonstrate regeneration (40). Although studies in animals show that most regeneration occurs within the first 2 weeks, this has not yet been proved in humans. Selective hepatic lobar hypertrophy is illustrated in Figure 5.
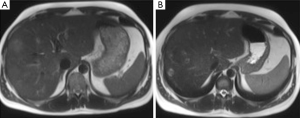
Outcomes
Technical success
The technical success rate should be close to 100%. Few cases of failures or repeated procedures have been reported in the literature (9,40). The resection rate should be approximately 85%. This rate may decrease to 70% in the case of cirrhotic patients. Reasons for non-resection are tumor progression, peritoneal metastases, or unsuspected metastases discovered at laparotomy. Absence of hypertrophy is rare, <10% in metastatic liver, but it can reach 20% in cirrhotic patients (19,20).
Hypertrophy response
CT, sometimes MRI, with volumetric is the cornerstone for planning surgical resection (19). There are different methods of calculating liver volumes, making comparison of results obtained at different institutions difficult. The growth of the FRL as a result of PVE can be calculated or expressed in two ways.
The difference in FRL volume before and after embolization in relation to the FRL volume before embolization (percentage volume increase):
FRL volume increase (%) = FRLpost-PVE (%) − FRLpre-PVE/FRLpre-PVE (%) × 100%
The difference between the percentage FRL before and after embolization [in literature referred to degree of hypertrophy (DH)]:
DH (%) = FRLpost-PVE (%) − FRLpre-PVE (%)
In patients with normal liver and liver metastases, the increase of the FLR ratio is between 8% and 25%, and regeneration is always observed after PVE. In cirrhotic patients, PVE fails to induce left-lobe hypertrophy in 20% of cases. Increased rate of FLR ratio in this population is slightly lower, between 6% and 20%.
Recent studies have demonstrated that hypertrophy is inversely proportional to the FRL ratio before PVE, meaning that the smaller FRL before PVE will have the larger hypertrophy (19,20). Consequently there is no lower limit for the FRL ratio to perform PVE.
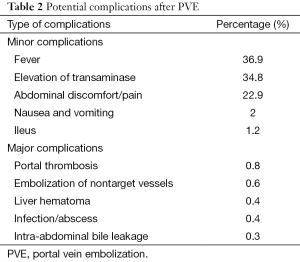
Complications
PVE is considerably less toxic than arterial embolization, so side effects are minimal. Signs and symptoms of postembolization syndrome, such as nausea and vomiting, are rare. Fever and pain are infrequent. Changes in liver function following PVE are usually minor and transient (50% of patients have no appreciable change) (Table 2). When transaminase levels rise, they usually peak at a level less than three times baseline 1-3 days after embolization and return to baseline in 7-10 days, regardless of the embolic materials used. Slight changes in total bilirubin value and white blood cell count may be seen. Synthetic function (prothrombin time) is almost never affected. PV thrombosis is extremely rare. Of course, it is essential to avoid the reflux of embolizing material into the portal venous branches of the remnant liver (13,21,40).
Full table
Unresolved issues regarding PVE
The purpose of PVE is to increase the hepatic functional reserve of FLR as well as its volume (41). However, there are four potential issues facing PVE: (I) PVE stimulates the growth of hepatic tumor (2,42,43); (II) PVE may fail to increase the volume of FLR in some patients, especially those with fibrotic or cirrhotic liver (3); (III) is PVE safe in patients with high-grade varices? The mechanisms of fast tumor growth after PVE are still poorly understood. Kokudo et al. (43) assessed the proliferative activity of intrahepatic metastases in the embolized liver after PVE in 18 patients with colorectal metastases and found a significantly increased tumor Ki-67 labeling index in the metastases group with PVE compared to hepatic metastases without PVE. It was postulated that the tumor growth after PVE might be controlled by three factors: malignant potential of the tumors, changes in cytokines or growth factors induced by PVE and changes in blood supply after PVE. Animal models of PV branch ligation demonstrated that HGF-mRNA markedly increased in the non-ligated growing lobe, but was only slightly elevated in the ligated shrinking lobe. Increased tissue levels of HGF might increase the level in plasma, thus stimulating the growth of hepatic tumors. Barbaro et al. (42) noted a significant increase in hepatic tumor volume from colorectal carcinoma after PVE, while hepatic tumor volume from carcinoid tumor was unchanged. Another factor potentially stimulating tumor growth after PVE is increased hepatic arterial blood flow in embolized liver after PVE, for supply of intrahepatic metastases depends solely on arterial blood supply (44). But these cannot explain why PVE increased hepatic tumor volume from colorectal carcinoma, while did not stimulate the growth of carcinoid tumor. Butyrate is known to stimulate proliferation of normal crypt cells, whereas it induces apoptosis and has antiangiogenic effects on colon cancer cells (45). Therefore, the lack of butyrate from PV blood may contribute to the increase in hepatic metastasis volume of colorectal carcinoma and, meanwhile, the enrichment of butyrate in FLR may help prevent tumor recurrence in patients treated with two-stage strategy. Hepatic arterial blood flow in embolized liver is increased after PVE and the supply of intrahepatic metastases depends solely on arterial blood supply, so PVE combined with transcatheter arterial embolization (TAE) may help prevent tumor growth and at the same time accelerate the hypertrophy of FLR. Pioneering reports from Inaba et al., and Sugawara et al., have confirmed that PVE in combination with TAE is safe, effective, and hence recommendable. PV pressure rises about 4 cm H2O after PVE (46), however, there is no report of PVE-related acute variceal hemorrhage. Liver transplantation is an excellent alternative to liver resection in treating the cirrhotic patient with small oligonodular hepatocellular carcinoma (HCC), but for large HCCs, partial liver resection remains the best therapeutic option for cure because neither liver transplantation nor percutaneous treatments are indicated. So PVE has become an important tool to induce hypertrophy of the FLR before major liver resection in cirrhotic patients (4); In PVE performed prior to an extended right hepatectomy, increasing attention has been given to embolization of segment IV. This embolization is performed for two reasons: (I) all tumor-bearing liver is embolized because accelerated tumor growth has been reported with incomplete embolization (47), and (II) segment IV embolization may contribute to better hypertrophy of segments I, II, and III before extended right hepatectomy (48). In addition, it is important to avoid reflux of the embolic material into the veins that will supply the FLR because bilateral or main PV occlusion remains a risk.
Conclusions
Preoperative PVE is an effective method to increase FRL volume with a high technical and clinical success rate. The complication rate is low, but local tumor progression after PVE is an imminent cause of unresectability. Pre-existing liver damage due to cirrhosis seems to have a negative effect on the hypertrophy response. Chemotherapy however does not seem to have any influence on the hypertrophy response, except for platin agents. The use of n-butyl cyanoacrylate may result in a greater hypertrophy response compared with the other embolization materials used.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Broering DC, Hillert C, Krupski G, Fischer L, Mueller L, Achilles EG, Schulte am Esch J, Rogiers X. Portal vein embolization vs. portal vein ligation for induction of hypertrophy of the future liver remnant. J Gastrointest Surg 2002;6:905-13; discussion 913. [PubMed]
- Hemming AW, Reed AI, Howard RJ, Fujita S, Hochwald SN, Caridi JG, Hawkins IF, Vauthey JN. Preoperative portal vein embolization for extended hepatectomy. Ann Surg 2003;237:686-91; discussion 691-3. [PubMed]
- Farges O, Belghiti J, Kianmanesh R, Regimbeau JM, Santoro R, Vilgrain V, Denys A, Sauvanet A. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg 2003;237:208-17. [PubMed]
- Jaeck D, Bachellier P, Nakano H, Oussoultzoglou E, Weber JC, Wolf P, Greget M. One or two-stage hepatectomy combined with portal vein embolization for initially nonresectable colorectal liver metastases. Am J Surg 2003;185:221-9. [PubMed]
- Liu H, Fu Y. Portal vein embolization before major hepatectomy. World J Gastroenterol 2005;11:2051-4. [PubMed]
- Couinaud C. Le foie: études anatomiques et chirurgicales. Paris, France: Masson, 1957;75.
- Schultz SR, LaBerge JM, Gordon RL, Warren RS. Anatomy of the portal vein bifurcation: intra- versus extrahepatic location--implications for transjugular intrahepatic portosystemic shunts. J Vasc Interv Radiol 1994;5:457-9. [PubMed]
- Yamane T, Mori K, Sakamoto K, Ikei S, Akagi M. Intrahepatic ramification of the portal vein in the right and caudate lobes of the liver. Acta Anat (Basel) 1988;133:162-72. [PubMed]
- Madoff DC, Hicks ME, Vauthey JN, Charnsangavej C, Morello FA Jr, Ahrar K, Wallace MJ, Gupta S. Transhepatic portal vein embolization: anatomy, indications, and technical considerations. Radiographics 2002;22:1063-76. [PubMed]
- Atri M, Bret PM, Fraser-Hill MA. Intrahepatic portal venous variations: prevalence with US. Radiology 1992;184:157-8. [PubMed]
- Hardy KJ, Jones RM. Failure of the portal vein to bifurcate. Surgery 1997;121:226-8. [PubMed]
- Vauthey JN, Chaoui A, Do KA, Bilimoria MM, Fenstermacher MJ, Charnsangavej C, Hicks M, Alsfasser G, Lauwers G, Hawkins IF, Caridi J. Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical associations. Surgery 2000;127:512-9. [PubMed]
- Abdalla EK, Hicks ME, Vauthey JN. Portal vein embolization: rationale, technique and future prospects. Br J Surg 2001;88:165-75. [PubMed]
- Shimamura T, Nakajima Y, Une Y, Namieno T, Ogasawara K, Yamashita K, Haneda T, Nakanishi K, Kimura J, Matsushita M, Sato N, Uchino J. Efficacy and safety of preoperative percutaneous transhepatic portal embolization with absolute ethanol: a clinical study. Surgery 1997;121:135-41. [PubMed]
- Lee KC, Kinoshita H, Hirohashi K, Kubo S, Iwasa R. Extension of surgical indications for hepatocellular carcinoma by portal vein embolization. World J Surg 1993;17:109-15. [PubMed]
- Kubota K, Makuuchi M, Kusaka K, Kobayashi T, Miki K, Hasegawa K, Harihara Y, Takayama T. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology 1997;26:1176-81. [PubMed]
- Azoulay D, Castaing D, Krissat J, Smail A, Hargreaves GM, Lemoine A, Emile JF, Bismuth H. Percutaneous portal vein embolization increases the feasibility and safety of major liver resection for hepatocellular carcinoma in injured liver. Ann Surg 2000;232:665-72. [PubMed]
- Nagino M, Nimura Y, Kamiya J, Kondo S, Uesaka K, Kin Y, Hayakawa N, Yamamoto H. Changes in hepatic lobe volume in biliary tract cancer patients after right portal vein embolization. Hepatology 1995;21:434-9. [PubMed]
- van Lienden KP, van den Esschert JW, de Graaf W, Bipat S, Lameris JS, van Gulik TM, van Delden OM. Portal vein embolization before liver resection: a systematic review. Cardiovasc Intervent Radiol 2013;36:25-34. [PubMed]
- Denys A, Bize P, Demartines N, Deschamps F, De Baere T. Cardiovascular and Interventional Radiological Society of Europe. Quality improvement for portal vein embolization. Cardiovasc Intervent Radiol 2010;33:452-6. [PubMed]
- de Baere T, Roche A, Elias D, Lasser P, Lagrange C, Bousson V. Preoperative portal vein embolization for extension of hepatectomy indications. Hepatology 1996;24:1386-91. [PubMed]
- Bruix J, Castells A, Bosch J, Feu F, Fuster J, Garcia-Pagan JC, Visa J, Bru C, Rodés J. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology 1996;111:1018-22. [PubMed]
- Farges O, Malassagne B, Flejou JF, Balzan S, Sauvanet A, Belghiti J. Risk of major liver resection in patients with underlying chronic liver disease: a reappraisal. Ann Surg 1999;229:210-5. [PubMed]
- Fujii Y, Shimada H, Endo I, Kamiyama M, Kamimukai N, Tanaka K, Kunisaki C, Sekido H, Togo S, Nagashima Y. Changes in clinicopathological findings after portal vein embolization. Hepatogastroenterology 2000;47:1560-3. [PubMed]
- Kusaka K, Imamura H, Tomiya T, Makuuchi M. Factors affecting liver regeneration after right portal vein embolization. Hepatogastroenterology 2004;51:532-5. [PubMed]
- Kakizawa H, Toyota N, Arihiro K, Naito A, Fujimura Y, Hieda M, Hirai N, Tachikake T, Matsuura N, Murakami Y, Itamoto T, Ito K. Preoperative portal vein embolization with a mixture of gelatin sponge and iodized oil: efficacy and safety. Acta Radiol 2006;47:1022-8. [PubMed]
- Nanashima A, Sumida Y, Abo T, Nonaka T, Takeshita H, Hidaka S, Sawai T, Yasutake T, Sakamoto I, Nagayasu T. Clinical significance of portal vein embolization before right hepatectomy. Hepatogastroenterology 2009;56:773-7. [PubMed]
- Covey AM, Brown KT, Jarnagin WR, Brody LA, Schwartz L, Tuorto S, Sofocleous CT, D'Angelica M, Getrajdman GI, DeMatteo R, Kemeny NE, Fong Y. Combined portal vein embolization and neoadjuvant chemotherapy as a treatment strategy for resectable hepatic colorectal metastases. Ann Surg 2008;247:451-5. [PubMed]
- van den Esschert JW, de Graaf W, van Lienden KP, Busch OR, Heger M, van Delden OM, Gouma DJ, Bennink RJ, Laméris JS, van Gulik TM. Volumetric and functional recovery of the remnant liver after major liver resection with prior portal vein embolization: recovery after PVE and liver resection. J Gastrointest Surg 2009;13:1464-9. [PubMed]
- Libicher M, Herbrik M, Stippel D, Poggenborg J, Bovenschulte H, Schwabe H. Portal vein embolization using the amplatzer vascular plug II: preliminary results. Rofo 2010;182:501-6. [PubMed]
- de Baere T, Teriitehau C, Deschamps F, Catherine L, Rao P, Hakime A, Auperin A, Goere D, Elias D, Hechelhammer L. Predictive factors for hypertrophy of the future remnant liver after selective portal vein embolization. Ann Surg Oncol 2010;17:2081-9. [PubMed]
- Giraudo G, Greget M, Oussoultzoglou E, Rosso E, Bachellier P, Jaeck D. Preoperative contralateral portal vein embolization before major hepatic resection is a safe and efficient procedure: a large single institution experience. Surgery 2008;143:476-82. [PubMed]
- Elias D, Ouellet JF, De Baère T, Lasser P, Roche A. Preoperative selective portal vein embolization before hepatectomy for liver metastases: long-term results and impact on survival. Surgery 2002;131:294-9. [PubMed]
- Nagino M, Kamiya J, Nishio H, Ebata T, Arai T, Nimura Y. Two hundred forty consecutive portal vein embolizations before extended hepatectomy for biliary cancer: surgical outcome and long-term follow-up. Ann Surg 2006;243:364-72. [PubMed]
- Liem MS, Liu CL, Tso WK, Lo CM, Fan ST, Wong J. Portal vein embolisation prior to extended right-sided hepatic resection. Hong Kong Med J 2005;11:366-72. [PubMed]
- Madoff DC, Abdalla EK, Gupta S, Wu TT, Morris JS, Denys A, Wallace MJ, Morello FA Jr, Ahrar K, Murthy R, Lunagomez S, Hicks ME, Vauthey JN. Transhepatic ipsilateral right portal vein embolization extended to segment IV: improving hypertrophy and resection outcomes with spherical particles and coils. J Vasc Interv Radiol 2005;16:215-25. [PubMed]
- Abulkhir A, Limongelli P, Healey AJ, Damrah O, Tait P, Jackson J, Habib N, Jiao LR. Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann Surg 2008;247:49-57. [PubMed]
- de Baere T, Denys A, Paradis V. Comparison of four embolic materials for portal vein embolization: experimental study in pigs. Eur Radiol 2009;19:1435-42. [PubMed]
- Loffroy R, Guiu B, Cercueil JP, Krausé D. Endovascular therapeutic embolisation: an overview of occluding agents and their effects on embolised tissues. Curr Vasc Pharmacol 2009;7:250-63. [PubMed]
- Madoff DC, Hicks ME, Abdalla EK, Morris JS, Vauthey JN. Portal vein embolization with polyvinyl alcohol particles and coils in preparation for major liver resection for hepatobiliary malignancy: safety and effectiveness--study in 26 patients. Radiology 2003;227:251-60. [PubMed]
- Kubo S, Shiomi S, Tanaka H, Shuto T, Takemura S, Mikami S, Uenishi T, Nishino Y, Hirohashi K, Kawamura E, Kinoshita H. Evaluation of the effect of portal vein embolization on liver function by (99m)tc-galactosyl human serum albumin scintigraphy. J Surg Res 2002;107:113-8. [PubMed]
- Barbaro B, Di Stasi C, Nuzzo G, Vellone M, Giuliante F, Marano P. Preoperative right portal vein embolization in patients with metastatic liver disease. Metastatic liver volumes after RPVE. Acta Radiol 2003;44:98-102. [PubMed]
- Kokudo N, Tada K, Seki M, Ohta H, Azekura K, Ueno M, Ohta K, Yamaguchi T, Matsubara T, Takahashi T, Nakajima T, Muto T, Ikari T, Yanagisawa A, Kato Y. Proliferative activity of intrahepatic colorectal metastases after preoperative hemihepatic portal vein embolization. Hepatology 2001;34:267-72. [PubMed]
- Kito Y, Nagino M, Nimura Y. Doppler sonography of hepatic arterial blood flow velocity after percutaneous transhepatic portal vein embolization. AJR Am J Roentgenol 2001;176:909-12. [PubMed]
- Zgouras D, Wächtershäuser A, Frings D, Stein J. Butyrate impairs intestinal tumor cell-induced angiogenesis by inhibiting HIF-1alpha nuclear translocation. Biochem Biophys Res Commun 2003;300:832-8. [PubMed]
- Ko GY, Sung KB, Yoon HK, Kim JH, Weon YC, Song HY. Preoperative portal vein embolization with a new liquid embolic agent. Radiology 2003;227:407-13. [PubMed]
- Elias D, De Baere T, Roche A. Mducreux, Leclere J, Lasser P. During liver regeneration following right portal embolization the growth rate of liver metastases is more rapid than that of the liver parenchyma. Br J Surg 1999;86:784-8. [PubMed]
- Nagino M, Nimura Y, Kamiya J, Kondo S, Uesaka K, Kin Y, Kutsuna Y, Hayakawa N, Yamamoto H. Right or left trisegment portal vein embolization before hepatic trisegmentectomy for hilar bile duct carcinoma. Surgery 1995;117:677-81. [PubMed]


