The value of delayed 18F FDG-PET imaging in diagnosis of solitary pulmonary nodules: A preliminary study on 28 patients
Abstract
Objective: The aim of this study was to investigate whether adding delayed phase imaging can improve diagnostic ability of 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) in evaluating solitary pulmonary nodules (SPNs).
Materials and methods: 28 patients with SPNs received dual-phase 18F-FDG PET at 1h and 2h after 18F-FDG injection during Feb 2009 to Jun 2011were included in this retrospective study. Their final diagnosis was confirmed by pathological examination in 27 cases and clinical follow-up in 1 case. The standardized uptake value (SUV) of early and delayed phases of all lesions was measured.
Results: The 28 SPNs included 9 benign lesions and 19 malignant lesions. Using SUV ≥2.5 as a criteria for malignancy, the sensitivity, specificity, and accuracy were 52.6%, 55.6% and 53.6% respectively at early phase; 68.4%, 55.6% and 64.3% respectively at early and delayed phases combined. Combined early and delayed phase scans combined picked up 3 additional malignant lesions from the 14 lesions with an initial SUV value less than 2.5, and there was no additional false positive result with the benign lesions.
Conclusions: Adding delayed phase scanning resulted in correct diagnosis of three malignant lesions with an initial SUV value less than 2.5. Delayed phase scanning can be recommended in the SPNs with SUV less than 2.5 at early phase.
Keywords
Solitary pulmonary nodules; 18F-FDG; SUV
Introduction
Diagnosis and management of solitary pulmonary nodules (SPNs) is a complex process and remains a challenge for both radiologists and physicians, even though CT and positron emission tomography (PET) or PET-CT are wildly used in evaluating these pulmonary lesions (1). The differential diagnosis of SPNs is extensive, including granuloma, hamartoma lung cancer, and metastasis (2). Once a SPN was detected, the first and most important goal of radiological evaluation is to noninvasively differentiate benign from malignant lesions as accurately as possible, because SPNs may be malignant and lung cancer has an overall mortality rate of up to 85% (3). On the other hand, malignant lesions account for only 60−80% of resected pulmonary nodules (4). 18F-fluorodeoxyglucose PET (18F-FDG PET) has been reported to be useful in characterizing SPNs (5,6). In the evaluation of an SPN, an SUV of ≥2.5 is frequently used as a criterion for malignancy. However, FDG is not a tumor-specific radiotracer, increased FDG uptake can be seen not only in malignancy but also seen in benign SPNs, while some malignant tumors, such as bronchoalveolar carcinoma and well differentiated adenocarcinoma, may exhibit only minimally increased activity (7-9). Many studies found that the FDG uptake value of a considerable amount of benign and malignant lesions overlaps (10-11). Some authors show that dual-time point imaging could improve the accuracy of FDG PET in the evaluation of lung lesions (12-15). The aim of this study was to assess whether adding delayed phase imaging can improve the accuracy of FDG PET in the evaluation of SPNs.
Materials and methods
Patients
Our institutional review board approved this retrospective study and waived informed consent. Twenty-eight patients with SPNs, receiving a dual-phase FDG PET from February 2009 to June 2011 in our institution, were enrolled in this study. There were 19 men and 9 women with age range of 33~85 years and average of 65.2 years. The final diagnosis was confirmed from pathologic examination (n=26), biopsy pathology (n=1), or repeated radiographic examination and clinical follow-up for more than 24 months (n=1).
FDG PET Scan
All scans were obtained on a dedicated whole-body PET/CT scanner (Biograph 64; Siemens). Before examination, informed consent for PET was obtained from all patients. At the time of FDG injection, all patients were instructed to fast for more than 6 hours and had fasting blood glucose levels of less than 140 mg/dL. The early phase image acquisition for the whole-body scan started at a mean time point of 60 minutes (range: 60−80 minutes) after injection of 5.18 MBq/kg of FDG per body weight. Patients were told to stay in a supine position in a solitary quiet environment for approximately 1 h waiting for the delayed imaging. Whole-body emission scan used 5 to 7 bed positions. PET used three dimensional (3D) acquisition. Acquisition time was 1.5~2.5 min per bed position. CT parameters were: effective amperage settings of 80~100 mAs; a tube voltage of 120 kV; a matrix of 512Χ512; a section thickness of 5 mm; and a reconstruction interval of 3 mm. Scanning was performed with the patients supine with arms raised above their heads. Delayed scan included one bed position in the thorax 110−150 minutes after tracer injection. A transmission scan was obtained with both sets of images for attenuation correction.
Image Interpretation
All PET/CT images were reviewed at a workstation with fusion software (syngo, Siemens) that displayed maximum intensity projection (MIP) PET images and multiplanar reformatted PET, CT and PET/CT fusion images. Two experienced diagnostic radiologists viewed the images in consensus. For semi-quantitative analysis, the maximal SUV of FDG was measured from both the early and delayed phase images by placing regions of interest (ROI) over the nodule that had the highest perceptible FDG uptake by visual analysis.
The accuracy for differentiating malignant nodules from benign nodules was calculated for the early, and early and delayed phases combined using SUV ≥2.5 as a criteria for malignancy, and clinical outcome served as reference standard.
Results
The 28 SPNs included 9 benign and 19 malignant nodules. In the nine benign nodules, there were 2 tuberculomas, 2 inflammatory granulomas, 3 non-specific inflammations, 1 cryptococcal infection, and 1 case of benign lesion without histropathologic evidence, which was regarded as benign by >24 months clinical follow-up. The malignant group consisted of 2 squamous cell carcinomas, 16 adenocarcinomas and one bronchoalveolar carcinoma containing a small amount of adenocarcinoma cells.
The sensitivity, specificity, and accuracy of the early phase in distinguishing malignancy from benign were 52.6%, 55.6% and 53.6%, respectively; while early and delayed phases combined achieved 68.4%,55.6% and 64.3%, respectively. Among the 14 nodules with SUV less than 2.5 at the early phase, SUV of 3 malignant lesions became higher than 2.5 at the delayed phase (Figure 1), while such change was not noted in the benign lesions (Figure 2). On the other hand, no lesions with SUV of 2.5 or higher at early phase experienced the change of SUV decreasing to less than 2.5 in the delayed phase. Therefore, combined early and delayed phase scans added three correct diagnosis cases in malignant lesions, while there was no additional false positive result with the benign lesions.

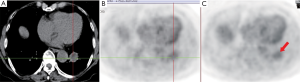
Discussion
Chest radiograph and CT screening have increased the detection of SPNs, but imaging evaluation of SPNs is a common diagnostic dilemma. It is essential to distinguish malignancy from benign nodules, because correct diagnosis can not only have small malignant SPNs resected at early stage to improve patients´ life quality and survive, but also avoid a benign node being unnecessary resected and reduce inappropriate invasive diagnostic investigation. With 18F-FDG PET, an SUV greater than 2.5 has been widely used as a diagnostic criteria of malignancy in evaluating SPNs with a sensitivity of 92−96% and a specificity of 77−90% (16-23).
However, SUV of benign and malignant lesions may have overlaps. Some malignant tumors, such as bronchoalveolar carcinoma and well differentiated adenocarcinoma, may exhibit only minimally increased activity, while some inflammatory lesions, such as pulmonary tuberculosis, can have high FDG uptake (7,8,10,11). Therefore, dual phase 18F-FDG PET has been proposed to evaluate pulmonary diseases, especially in the lesions with initial SUV less than 2.5 (9,12-15). The reported results varied. Some studies showed that dual phase imaging could improve the accuracy of PET in distinguishing between benign and malignant disease due to that malignancies tend to retain the tracer (12-14), but some studies´ results did not support this finding (9,24,25). Chen et al used dual-phase FDG PET to evaluate pulmonary nodules with an initial SUV less than 2.5 and found that dual phase FDG PET could not improve the diagnosis performance of PET on pulmonary diseases in geographic regions with high risk of granulomatous inflammation (9).
In our study, we added a 120-minute delayed imaging to the initial imaging at 60 minutes in 28 patients to evaluate SPNs. The aim of our study was to examine whether adding delayed phase imaging can improve diagnosis ability of PET in assessment of SPNs. Using SUV≥2.5 as the criteria for malignancy, combined early phase and delayed phase scans obtained an accuracy of 64.3% (sensitivity: 68.4%; specificity: 55.6%), which was slight higher than the accuracy of 53.6% (sensitivity: 52.6%; specificity: 55.6%) at the early phase scan only. Our results were consistent with what Chen and his colleagues reported (9). However, in our series, three malignant SPNs with initial SUV less than 2.5 were measured with SUV higher than 2.5 at delayed phase, while no benign lesions experienced such changes and no malignancy with SUV more than 2.5 at initial imaging alternated their SUV to less than 2.5. Therefore, the early and delayed phase combined added three true positive cases, and the sensitivity increased without sacrifice of specificity. Considering benefits of the three patients obtained, it´s worthy to carry out the delayed phase imaging in those SPNs with initial SUV less than 2.5 at the early phase.
False-positive result occurred in 4 benign nodules in our series which had resulted in an unnecessary lobectomy or wedge resection. These nodules were incorrectly considered to be malignancy because they all showed high uptake of FDG (SUV >2.5) at early phase. Similar false-positive findings have been reported (9,26-27). The result indicated delayed phase FDG PET imaging may not eliminate unnecessary invasive procedures for benign lesions completely, and positive SPNs should be cautiously interpreted.
In summary, three patients with malignant SPNs benefited from adding delayed phase imaging in our present study group of 28 SPNs. Considering advantage the correct diagnosis brought to the three patients, delayed phase scanning can be recommended in the SPNs with SUV less than 2.5 at early phase.
References
- Jeong YJ, Yi CA, Lee KS. Solitary pulmonary nodules: detection, characterization, and guidance for further diagnostic workup and treatment. AJR Am J Roentgenol 2007;188:57-68.[LinkOut]
- Winer-Muram HT. The solitary pulmonary nodule. Radiology 2006;239:34-49.[LinkOut]
- Murthy SC, Rice TW. The solitary pulmonary nodule: a primer on differential diagnosis. Semin Thorac Cardiovasc Surg 2002;14:239-49.[LinkOut]
- Midthun DE, Swensen SJ, Jett JR. Approach to the solitary pulmonary nodule. Mayo Clin Proc 1993;68:378-85.[LinkOut]
- Vazquez M, Carter D, Brambilla E, et al. Solitary and multiple resected adenocarcinomas after CT screening for lung cancer: histopathologic features and their prognostic implications. Lung Cancer 2009;64:148-54.[LinkOut]
- Varoli F, Vergani C, Caminiti R, et al. Management of solitary pulmonary nodule. Eur J Cardiothorac Surg 2008;33:461-5.[LinkOut]
- Kapucu LO, Meltzer CC, Townsend DW, et al. Fluorine-18-fluorodeoxyglucose uptake in pneumonia. J Nucl Med 1998;39:1267-9.[LinkOut]
- Nomori H, Watanabe K, Ohtsuka T, et al. Evaluation of F-18 fluorodeoxyglucose (FDG) PET scanning for pulmonary nodules less than 3 cm in diameter, with special reference to the CT images. Lung Cancer 2004;45:19-27.[LinkOut]
- Chen CJ, Lee BF, Yao WJ, et al. Dual-phase 18F-FDG PET in the diagnosis of pulmonary nodules with an initial standard uptake value less than 2.5. AJR Am J Roentgenol 2008;191:475-9.[LinkOut]
- Higashi K, Ueda Y, Seki H, et al. Fluorine-18-FDG PET imaging is negative in bronchioloalveolar lung carcinoma. J Nucl Med 1998;39:1016-20.[LinkOut]
- Ichiya Y, Kuwabara Y, Sasaki M, et al. FDG-PET in infectious lesions: The detection and assessment of lesion activity. Ann Nucl Med 1996;10:185-91.[LinkOut]
- Matthies A, Hickeson M, Cuchiara A, et al. Dual time point 18F-FDG PET for the evaluation of pulmonary nodules. J Nucl Med 2002;43:871-5.[LinkOut]
- Zhuang H, Pourdehnad M, Lambright ES, et al. Dual time point 18F-FDG PET imaging for differentiating malignant from inflammatory processes. J Nucl Med 2001;42:1412-7.[LinkOut]
- Xiu Y, Bhutani C, Dhurairaj T, et al. Dual-time point FDG PET imaging in the evaluation of pulmonary nodules with minimally increased metabolic activity. Clin Nucl Med 2007;32:101-5.[LinkOut]
- Lin WY, Tsai SC, Hung GU. Value of delayed 18F-FDG-PET imaging in the detection of hepatocellular carcinoma. Nucl Med Commun 2005;26:315-21.[LinkOut]
- Gould MK, Maclean CC, Kuschner WG, et al. Accuracy of positron emission tomography for diagnosis of pulmonary nodules and mass lesions: a meta-analysis. JAMA 2001;285:914-24.[LinkOut]
- Cronin P, Dwamena BA, Kelly AM, et al. Solitary pulmonary nodules: meta-analytic comparison of cross-sectional imaging modalities for diagnosis of malignancy. Radiology 2008;246:772-82.[LinkOut]
- Conti PS, Lilien DL, Hawley K, et al. PET and [18F]-FDG in oncology: a clinical update. Nucl Med Biol 1996;23:717-35.[LinkOut]
- Gupta NC, Frank AR, Dewan NA, et al. Solitary pulmonary nodules: detection of malignancy with PET with 2-[F-18]-fluoro-2-deoxy-D-glucose. Radiology 1992;184:441-4.[LinkOut]
- Gupta NC, Maloof J, Gunel E. Probability of malignancy in solitary pulmonary nodules using fluorine-18-FDG and PET. J Nucl Med 1996;37:943-8.[LinkOut]
- Hübner KF, Buonocore E, Gould HR, et al. Differentiating benign from malignant lung lesions using "quantitative" parameters of FDG PET images. Clin Nucl Med 1996;21:941-9.[LinkOut]
- Lowe VJ, Fletcher JW, Gobar L, et al. Prospective investigation of positron emission tomography in lung nodules. J Clin Oncol 1998;16:1075-84.[LinkOut]
- Scott WJ, Schwabe JL, Gupta NC, et al. Positron emission tomography of lung tumors and mediastinal lymph nodes using [18F]fluorodeoxyglucose. The Members of the PET-Lung Tumor Study Group. Ann Thorac Surg 1994;58:698-703.[LinkOut]
- Yen TC, Chang YC, Chan SC, et al. Are dual-phase 18F-FDG PET scans necessary in nasopharyngeal carcinoma to assess the primary tumour and loco-regional nodes? Eur J Nucl Med Mol Imaging 2005;32:541-8.[LinkOut]
- Döbert N, Hamscho N, Menzel C, et al. Limitations of dual time point FDG-PET imaging in the evaluation of focal abdominal lesions. Nuklearmedizin 2004;43:143-9.[LinkOut]
- Laffon E, de Clermont H, Begueret H, et al. Assessment of dual-time-point 18F-FDG-PET imaging for pulmonary lesions. Nucl Med Commun 2009;30:455-61.[LinkOut]
- Demura Y, Tsuchida T, Ishizaki T, et al. 18F-FDG accumulation with PET for differentiation between benign and malignant lesions in the thorax. J Nucl Med 2003;44:540-8.[LinkOut]


