Quantifying the deficit—imaging neurobehavioural impairment in childhood epilepsy
Introduction
It is acknowledged that there is a high incidence of neurobehavioural disorders in children with epilepsy (1) and that these associated cognitive, behavioural and psychological impairments can be equally, if not more disabling than the epileptic seizures themselves. Studies of quality of life and long term social outcomes in children with epilepsy have shown that the presence and severity of neurobehavioural impairments is a stronger predictor of long term outcome than seizure control or other epilepsy related factors (2,3). Nevertheless they remain an often overlooked part of epilepsy management (4,5).
There is now an extensive literature showing that the incidence of several neuropsychiatric/neurobehavioural disorders, including depression (6), learning disability (LD) (7), attention deficit hyperactivity disorder (ADHD) (8) and autistic spectrum disorder (ASD) (9) is significantly increased in children with epilepsy over and above that seen in healthy children or children with other childhood illnesses such as migraine, diabetes or asthma (5,10-13). However, our understanding of the underlying neurological basis for this association remains in its infancy. Many of these impairments have been shown to be present at or pre-date seizure onset (14-17), moreover, the relationship between epilepsy-related factors such as seizure control, treatment or epilepsy syndrome and neurobehavioural impairments is not straightforward (12,18-21). One conclusion from this is that, while seizures may play a role, they are unlikely to be the only pathological factor and attention needs to be focused on underlying abnormalities that may be responsible for both the impairment and the epilepsy together.
The majority of children with epilepsy, including those with neurobehavioural impairments, do not have visible abnormalities on neuroimaging (22). While improvements in imaging technology may allow the visualisation of hitherto undetected macroscopic abnormalities (23) this will still remain a minority of patients. The use of advanced neuroimaging techniques such as automated cortical thickness/volume measurements, functional magnetic resonance imaging (fMRI) and diffusion tensor imaging (DTI) has the ability to provide information on changes to brain microstructure, connectivity and organisation over and above that available from conventional imaging and there are now a number of studies showing that children with epilepsy show subtle differences in grey matter volumes, white matter integrity and network connectivity compared to healthy control groups (24). There is growing interest in relating these concepts to neurobehavioural impairments in children with epilepsy and it is hoped that this may provide further insight into the neurological basis of these impairments and their underlying causes. By understanding the “neural signature” of these conditions it may then be possible to identify biomarkers to assist with early diagnosis and risk stratification.
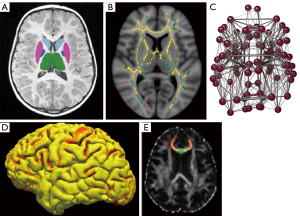
One barrier is that quantitative magnetic resonance imaging (MRI) techniques, including volumetry, cortical thickness measurements, DTI, fMRI or structural/functional network analysis (Figure 1) have rarely been systematically applied in children with epilepsy. Furthermore, those studies that have been carried out have tended to focus on specific subtypes of epilepsy and specific neurobehavioural impairments. Since there is a tendency for impairments to cluster and frequently occur in association with one another there is a need to consider the evidence in toto to identify common factors. The purpose of this review is to try and draw together the disparate threads of evidence from studies looking at different impairments and comorbidities to assess the utility of different quantitative MRI techniques to detect and understand their common foundation.
Methods
Articles relevant to this review were identified by searches of the PubMed (National Center for Biotechnology Information), Medline/OvidSP, Web-of-Science and BIOSIS databases, performed on 17th August 2014. Medical Subject Heading (MeSH) terms “Epilepsy” (including all sub-categories) and “MR”, “diffusion tensor imaging”, “diffusion magnetic resonance imaging”, “neuroimaging” were combined with appropriate terms for different specific impairments as follows:
- “attention deficit hyperactivity disorder”, “attention deficit disorder”, “attention deficit disorder with hyperactivity”;
- “autism”, “autistic spectrum disorder”, “Asperger’s syndrome”, “Kanner’s syndrome”, “autistic disorder”, “infantile autism”;
- “cognition”, “cognitive disorders”, “learning difficulty”, “mental retardation”, “intellectual impairment”;
- “behavioural problems”, “personality disorder”, “oppositional defiant disorder”;
- “mood disorders”, “anxiety”, “depression”;
- “neurobehavioural impairment”, “comorbidity”, “neurodevelopmental impairment”, “neuropsychological impairment”.
Searches were limited to original research studies in English involving children under the age of 18. In addition references from relevant original articles and review articles were added if not identified from these searches. The titles and abstracts of all articles identified from these searches and where necessary to make a decision on inclusion, the full text article were retrieved and reviewed. Criteria applied for consideration of inclusion in this review were that the article should have reported on the results of an original research study involving neuroimaging in patients with epilepsy and one of the above-mentioned impairments with comparison to a suitable control group, and that the study should include a group of children under the age of 18 years and report separately on this group. Studies with a mixed group of children and adults were not included unless there was a separate analysis only including the child group. Studies that focused exclusively on children post-epilepsy surgery were excluded.
Results
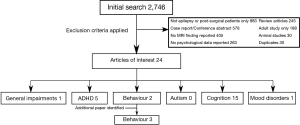
From the initial search 2,746 articles were identified. Of these 24 were considered applicable to this review. The majority of the excluded articles either did not address MRI or neurobehavioural findings, contained only single case reports or reviews of existing data, did not report neuroimaging in a systematic way or only included children with epilepsy incidentally and did not report on them as a specific group. Figure 2 shows how the identified articles were categorised by type of impairment. One additional article was identified from references and is described below.
Attention deficit hyperactivity disorder (ADHD)
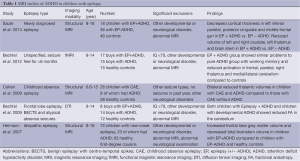
Five articles investigating ADHD in children with epilepsy were considered applicable to this review. These were split into two main approaches. Studies are summarised in Table 1.
Full table
In two separate studies, Bechtel et al. found that children with epilepsy and ADHD showed similar patterns of change to children with developmental ADHD (and no epilepsy) with reduced activation within frontal, parietal and cerebellar regions on fMRI during working memory tasks (25) and reduced fractional anisotropy in the middle cerebellar peduncle on DTI (26). Their conclusion was that the pattern of neurological involvement in epilepsy and ADHD was similar to that seen in ADHD alone, although no children with epilepsy without ADHD were included in either study for comparison.
Conversely three studies by a group led by Hermann compared children with epilepsy alone to children with epilepsy and ADHD but did not include a group of children without epilepsy but with ADHD for comparison. They found significant reductions in thalamic volumes (27) and frontal lobe grey matter and brainstem volumes (14) in children with epilepsy with ADHD compared to those with epilepsy alone and with normal controls, findings that have not previously been reported in children with idiopathic ADHD. Most recently (28) they report reduced cortical thickness measurements in specific areas within bilateral frontal, temporal and parietal lobes, as well as reduced right basal ganglia and thalamic volumes in children with epilepsy and ADHD compared to those with epilepsy alone.
Despite the disparate methodologies of these studies; two looking predominantly at ADHD with and without epilepsy and three at epilepsy with and without ADHD and the variety of MRI techniques employed; the conclusions reported are in keeping with those reported in studies of idiopathic ADHD, which have shown disruption within a broad fronto-striatal-cerebellar network (29) and similar patterns of cortical thinning (30).
Whether or not there is a unique phenotype to epilepsy-associated ADHD remains a matter of debate, with some reports of an increase in inattentive-type ADHD compared to idiopathic ADHD (14); subtle differences may exist in the neural substrates, with more widespread involvement of the thalamus and brainstem in children with epilepsy-associated ADHD, but overall the imaging changes demonstrated to date appear broadly similar. Interestingly similar fMRI have been reported in adult studies of working memory in patients with temporal lobe epilepsy (31) raising the possibility that these findings may have wider relevance.
Autism
No systematic neuroimaging studies involving patients with autism and epilepsy were identified. A number of studies described MRI findings in patients with tuberous sclerosis (with and without epilepsy) suggesting that the type and location of tubers might be linked with the risk of epilepsy and autistic behaviour (32-34), but none of these included a control group and they did not elaborate on any link between the autism and the epilepsy. All other studies either did not review any neuroimaging or presented isolated case reports.
The suggestion has been made that the increased risk of autism in children with epilepsy can be mainly explained by the increased incidence of learning difficulty rather than being linked to the epilepsy itself (9,35). Currently little evidence exists to refute this hypothesis, although the association of autism with specific epilepsy syndromes such as infantile spasms does suggest that there may still be specific mechanisms at work.
Cognition
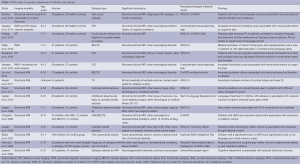
There were 15 articles identified that investigated the relationship between one or more aspects of cognition, epilepsy and brain structure. These articles covered a number of different epilepsy syndromes and investigated a number of different cognitive aspects and are therefore difficult to compare directly. Findings are summarised in Table 2.
Full table
The most common types of epilepsy to be studied were benign epilepsy with centro-temporal spikes (BECTS), childhood absence epilepsy (CAE) and idiopathic epilepsy with complex partial seizures (CPS). The majority of studies, apart from one early study by Lawson et al. (36), used validated neuropsychological tests to assess cognitive function. The main assessment tools used were the WISC-III (37) for general cognition/intelligence and the Delis-Kaplan Executive Function System (38) for executive function, with single studies using other tools such as the Test of Language Development (39) and the Children’s Memory Scale (40). MRI investigations were more heterogeneous, with a number of different structural and functional imaging techniques used and little overlap between individual studies.
In general abnormal structural neuroimaging (41,42) and, in particular, a loss of cortical grey matter volume (43-47) was associated with poorer cognitive performance/cognitive impairment. There is however little agreement as to the cortical areas involved, with different studies reporting changes within frontal (44), temporal (48) and parieto-occipital (45) lobes. Poorer executive function was associated with changes within the basal ganglia and thalami (49,50) and with reductions in temporal white matter integrity (51). Evidence from DTI and fMRI suggests that decreased white matter integrity along fronto-occipital tracts (52) as well as within the corpus callosum (51) and disrupted brain networks (53-55) may also be associated with impaired performance on cognitive and language tasks. One implication is that cognitive impairment in children with epilepsy may not be associated with alterations within a single brain region but a product of changes in anatomically diverse brain areas and affecting both grey and white matter.
This parallels the literature on cognitive ability in the general population which has also been linked to both grey matter volume (56) as well as white matter integrity (57). It has been suggested that cognitive ability is a distributed property of the brain and that white matter network connectivity is a key component (58,59) with more “efficient” network architectures associated with improved cognitive ability.
Behaviour
Two studies were identified from the initial search that looked at behavioural problems and neuroimaging in children with epilepsy and were considered applicable to this review (Table 3). These two studies, from the same group in Japan, looked at children with frontal lobe epilepsy (60) and BECTS (61), with and without behavioural problems and cognitive co-morbidity. However the limited number of patients with epilepsy and behavioural problems in each study (two patients in each study) preclude any formal statistical analysis being presented. In these two small longitudinal studies reduced frontal and prefrontal lobe growth was shown to be reduced in children with frontal lobe epilepsy (FLE)/BECTS and behavioural and cognitive problems over a 2-year period after initial presentation compared to children with FLE/BECTS and no impairments.
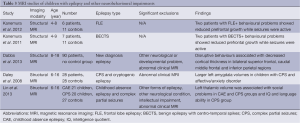
Full table
One additional study was identified from other references. This study, using the child-behaviour checklist, of 90 children with new-onset epilepsy found that higher social competence was associated with increased cortical thickness in the left superior frontal and left postcentral regions, whereas disruptive behavioural problems were associated with decreased cortical thickness predominantly in bilateral frontal regions and internalising behavioural problems (somatic disturbances, affective disorder, anxiety) were associated with fewer significant changes (62). No healthy control group was included and raw behavioural scores were not reported, so it is not possible to ascertain if these children were displaying more problems than the general population, although it is known that children with new-onset epilepsy are reported to show more problems than unaffected siblings using this checklist (16).
The evidence for a structural link between epilepsy and behavioural problems remains weak and no controlled studies have been performed; the existing data suggests that disruption of frontal lobe development may be a factor.
Mood disorders
Only one study was identified in the search that was considered applicable to this review (Table 3). Despite the substantial literature on amygdala/basal ganglia abnormalities and depression in adults with temporal lobe epilepsy (TLE) (63), little systematic study appears to have been carried out in a paediatric age group. The single study that has been performed (64) looked at children with active CPS and found that larger amygdala volumes were associated with a higher risk of affective disorder, a similar finding to that reported in the adult TLE literature (63) but in contrast to studies of major depressive disorder (65). Although they were unable to control effectively for IQ this raises the possibility that affective disorder in epilepsy may be distinct to mood disorders found in children without seizures and have a different pathophysiological basis.
Neurobehavioural impairments
One additional article by Lin et al. (66) was identified that looked at neurobehavioural impairments as a group in children with CAE and CPS (Table 3). They found an association between decreasing left thalamic volume and higher social problem scores for both epilepsy groups and a further association with lower IQ and language scores for children with CPS.
Discussion
Overall, there are a limited number of studies looking at the neuroimaging associations of neurobehavioural impairment in children with epilepsy. Most studies focus on either one particular type of epilepsy or a single impairment or both. There are almost no studies looking at younger children under the age of 5 years and data looking at children with multiple impairments or significant learning difficulties is likewise extremely limited. The majority of studies exclude children with other neurological problems, known developmental problems or abnormalities on routine neuroimaging. Part of the reason may be because this group of children often have multiple medical/developmental comorbidities and are likely to require general anaesthesia for neuroimaging investigations, complicating their assessment. Exclusion of these children is however problematic as it is known that a high proportion of children with epilepsy have seizures caused by a known neurological condition or structural brain abnormality and that the clustering effect of neurobehavioural impairments mean that comorbidity is likely to be common. As analysis techniques improve, structural or neurological abnormalities need not be a priori grounds for exclusion, although technical refinements may be required. Therefore despite the methodological difficulties associated with working with this group of children it will be important that attempts be made to include them in future studies.
Cognitive impairment and executive function are the best studied impairments. There is now evidence from a number of studies that structural brain changes are present at or near to epilepsy diagnosis and that these changes can be associated with deleterious effects on cognition. The diversity of imaging methods reported make synthesis difficult: there is a general trend to the implication of multiple, anatomically distinct brain areas, but little agreement on which areas are important. Increasing evidence from advanced imaging techniques raises the interesting suggestion that the primary problem may be one of abnormal white matter connectivity and network disruption, rather than grey matter loss.
The five studies on ADHD in children with epilepsy also report the involvement of multiple cortical areas, with changes also noted in some subcortical structures such as the thalamus and brainstem. Other impairments are less well studied, with the scarce evidence available coming either from single, small studies or uncontrolled observational cohorts.
In summary, the small number of studies and disparate methodology make synthesis of the structural brain changes in epilepsy associated impairments difficult. There is a strong sense that network dysfunction is important, and that therefore imaging techniques that can assess this directly, such as fMRI and DTI are more likely to provide useful information, but the extent to which findings can be generalised across different epileptic syndromes and aetiologies remains to be determined. Likewise it remains uncertain the extent to which impairments in children with epilepsy differ from similar impairments in children without seizures. In view of the large number of epilepsy syndromes known, it will be important to understand how neurobehavioural impairments arise across different forms of epilepsy, especially those associated with early onset, structural lesions or multiple impairments. Ideally studies should assess children as near to diagnosis as possible to avoid confounding from the effects of seizures and medication. As neurobehavioural impairments have been found across almost all epilepsy syndromes, it will be important to move from studying isolated syndromes to broader studies including a range of different types of epilepsy. Studies focusing on these groups at greatest risk of impairments and with more severe impairments, for example on children with early onset epilepsy or with specific aetiological classifications, will be crucial in this regard. By including children with more severe and multiple impairments, although the power to detect syndrome specific changes will be lost, paradoxically power to detect associated structural brain changes may improve as more severe changes will be expected. Since many analysis techniques are still developing, the collection of a broad range of MRI data that will be able to accommodate future advances and analysis will be important to allow further information to be gained from existing cohorts as our understanding of these conditions advances. Correlating this with high quality neurocognitive and clinical data remains the key to unlocking the underlying neurological basis of these conditions and improving their diagnosis and treatment.
Regardless of methodology, the increasing awareness of neurobehavioural impairments and introduction of advanced quantitative neuroimaging techniques to traditional epilepsy cohorts mean that, while this is currently still a relatively unexplored area, it is one that is likely to undergo rapid expansion.
Disclosure: The author declares no conflict of interest.
References
- Kerr MP, Mensah S, Besag F, de Toffol B, Ettinger A, Kanemoto K, Kanner A, Kemp S, Krishnamoorthy E, LaFrance WC Jr, Mula M, Schmitz B, van Elst LT, Trollor J, Wilson SJ; International League of Epilepsy (ILAE) Commission on the Neuropsychiatric Aspects of Epilepsy. International consensus clinical practice statements for the treatment of neuropsychiatric conditions associated with epilepsy. Epilepsia 2011;52:2133-8. [PubMed]
- Camfield CS, Camfield PR. Long-term social outcomes for children with epilepsy. Epilepsia 2007;48:3-5. [PubMed]
- Baca CB, Vickrey BG, Caplan R, Vassar SD, Berg AT. Psychiatric and medical comorbidity and quality of life outcomes in childhood-onset epilepsy. Pediatrics 2011;128:e1532-43.
- Hamiwka LD, Wirrell EC. Comorbidities in pediatric epilepsy: beyond "just'' treating the seizures. J Child Neurol 2009;24:734-42. [PubMed]
- Reilly C, Atkinson P, Das KB, Chin RF, Aylett SE, Burch V, Gillberg C, Scott RC, Neville BG. Neurobehavioral comorbidities in children with active epilepsy: a population-based study. Pediatrics 2014;133:e1586-93.
- Reilly C, Agnew R, Neville BG. Depression and anxiety in childhood epilepsy: a review. Seizure 2011;20:589-97. [PubMed]
- Rantanen K, Eriksson K, Nieminen P. Cognitive impairment in preschool children with epilepsy. Epilepsia 2011;52:1499-505. [PubMed]
- Chou IC, Chang YT, Chin ZN, Muo CH, Sung FC, Kuo HT, Tsai CH, Kao CH. Correlation between epilepsy and attention deficit hyperactivity disorder: a population-based cohort study. PLoS One 2013;8:e57926. [PubMed]
- Berg AT, Plioplys S, Tuchman R. Risk and correlates of autism spectrum disorder in children with epilepsy: a community-based study. J Child Neurol 2011;26:540-7. [PubMed]
- Rai D, Kerr MP, McManus S, Jordanova V, Lewis G, Brugha TS. Epilepsy and psychiatric comorbidity: a nationally representative population-based study. Epilepsia 2012;53:1095-103. [PubMed]
- Selassie AW, Wilson DA, Martz GU, Smith GG, Wagner JL, Wannamaker BB. Epilepsy beyond seizure: a population-based study of comorbidities. Epilepsy Res 2014;108:305-15. [PubMed]
- Berg AT, Caplan R, Hesdorffer DC. Psychiatric and neurodevelopmental disorders in childhood-onset epilepsy. Epilepsy Behav 2011;20:550-5. [PubMed]
- Davies S, Heyman I, Goodman R. A population survey of mental health problems in children with epilepsy. Dev Med Child Neurol 2003;45:292-5. [PubMed]
- Hermann B, Jones J, Dabbs K, Allen CA, Sheth R, Fine J, McMillan A, Seidenberg M. The frequency, complications and aetiology of ADHD in new onset paediatric epilepsy. Brain 2007;130:3135-48. [PubMed]
- Oostrom KJ, Smeets-Schouten A, Kruitwagen CL, Peters AC, Jennekens-Schinkel A; Dutch Study Group of Epilepsy in Childhood. Not only a matter of epilepsy: early problems of cognition and behavior in children with "epilepsy only"--a prospective, longitudinal, controlled study starting at diagnosis. Pediatrics 2003;112:1338-44. [PubMed]
- Austin JK, Harezlak J, Dunn DW, Huster GA, Rose DF, Ambrosius WT. Behavior problems in children before first recognized seizures. Pediatrics 2001;107:115-22. [PubMed]
- Loney JC, Wirrell EC, Sherman EM, Hamiwka LD. Anxiety and depressive symptoms in children presenting with a first seizure. Pediatr Neurol 2008;39:236-40. [PubMed]
- Smith ML, Elliott IM, Lach L. Cognitive, psychosocial, and family function one year after pediatric epilepsy surgery. Epilepsia 2004;45:650-60. [PubMed]
- Viggedal G, Olsson I, Carlsson G, Rydenhag B, Uvebrant P. Intelligence two years after epilepsy surgery in children. Epilepsy Behav 2013;29:565-70. [PubMed]
- Masur D, Shinnar S, Cnaan A, Shinnar RC, Clark P, Wang J, Weiss EF, Hirtz DG, Glauser TA; Childhood Absence Epilepsy Study Group. Pretreatment cognitive deficits and treatment effects on attention in childhood absence epilepsy. Neurology 2013;81:1572-80. [PubMed]
- Braakman HM, Ijff DM, Vaessen MJ, Debeij-van Hall MH, Hofman PA, Backes WH, Vles JS, Aldenkamp AP. Cognitive and behavioural findings in children with frontal lobe epilepsy. Eur J Paediatr Neurol 2012;16:707-15. [PubMed]
- Berg AT, Testa FM, Levy SR, Shinnar S. Neuroimaging in children with newly diagnosed epilepsy: A community-based study. Pediatrics 2000;106:527-32. [PubMed]
- Winston GP, Micallef C, Kendell BE, Bartlett PA, Williams EJ, Burdett JL, Duncan JS. The value of repeat neuroimaging for epilepsy at a tertiary referral centre: 16 years of experience. Epilepsy Res 2013;105:349-55. [PubMed]
- Hermann BP, Lin JJ, Jones JE, Seidenberg M. The emerging architecture of neuropsychological impairment in epilepsy. Neurol Clin 2009;27:881-907. [PubMed]
- Bechtel N, Kobel M, Penner IK, Specht K, Klarhöfer M, Scheffler K, Opwis K, Schmitt-Mechelke T, Capone A, Weber P. Attention-deficit/hyperactivity disorder in childhood epilepsy: a neuropsychological and functional imaging study. Epilepsia 2012;53:325-33. [PubMed]
- Bechtel N, Kobel M, Penner IK, Klarhöfer M, Scheffler K, Opwis K, Weber P. Decreased fractional anisotropy in the middle cerebellar peduncle in children with epilepsy and/or attention deficit/hyperactivity disorder: a preliminary study. Epilepsy Behav 2009;15:294-8. [PubMed]
- Schreibman Cohen A, Daley M, Siddarth P, Levitt J, Loesch IK, Altshuler L, Ly R, Shields WD, Gurbani S, Caplan R. Amygdala volumes in childhood absence epilepsy. Epilepsy Behav 2009;16:436-41. [PubMed]
- Saute R, Dabbs K, Jones JE, Jackson DC, Seidenberg M, Hermann BP. Brain morphology in children with epilepsy and ADHD. PLoS One 2014;9:e95269. [PubMed]
- De La Fuente A, Xia S, Branch C, Li X. A review of attention-deficit/hyperactivity disorder from the perspective of brain networks. Front Hum Neurosci 2013;7:192. [PubMed]
- Narr KL, Woods RP, Lin J, Kim J, Phillips OR, Del'Homme M, Caplan R, Toga AW, McCracken JT, Levitt JG. Widespread cortical thinning is a robust anatomical marker for attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2009;48:1014-22. [PubMed]
- Stretton J, Winston G, Sidhu M, Centeno M, Vollmar C, Bonelli S, Symms M, Koepp M, Duncan JS, Thompson PJ. Neural correlates of working memory in Temporal Lobe Epilepsy--an fMRI study. Neuroimage 2012;60:1696-703. [PubMed]
- Bolton PF, Griffiths PD. Association of tuberous sclerosis of temporal lobes with autism and atypical autism. Lancet 1997;349:392-5. [PubMed]
- Curatolo P, Porfirio MC, Manzi B, Seri S. Autism in tuberous sclerosis. Eur J Paediatr Neurol 2004;8:327-32. [PubMed]
- Gallagher A, Grant EP, Madan N, Jarrett DY, Lyczkowski DA, Thiele EA. MRI findings reveal three different types of tubers in patients with tuberous sclerosis complex. J Neurol 2010;257:1373-81. [PubMed]
- Amiet C, Gourfinkel-An I, Bouzamondo A, Tordjman S, Baulac M, Lechat P, Mottron L, Cohen D. Epilepsy in autism is associated with intellectual disability and gender: evidence from a meta-analysis. Biol Psychiatry 2008;64:577-82. [PubMed]
- Lawson JA, Cook MJ, Bleasel AF, Nayanar V, Morris KF, Bye AM. Quantitative MRI in outpatient childhood epilepsy. Epilepsia 1997;38:1289-93. [PubMed]
- Shaw SR, Swerdlik ME, Laurent J. Review of the WISC-III. In: Bracken BA, McCallum RS. eds. Wechsler Intelligence Scale for Children Third edition Journal of Psychoeducational Assessment Advances in psychoeducational assessment. Brandon, VT: Clinical Psychology Publishing Co, 1993:151-60.
- Delis DC, Kramer JH, Kaplan E, Holdnack J. Reliability and validity of the Delis-Kaplan Executive Function System: an update. J Int Neuropsychol Soc 2004;10:301-3. [PubMed]
- Newcomer P, Hammill DD. Using the test of language development with language-impaired children. J Learn Disabil 1978;11:521-4. [PubMed]
- Cohen M. eds. Children’s Memory Scale: Administration Manual. San Antonio, Texas: The Psychological Corporation, 1997.
- Byars AW, deGrauw TJ, Johnson CS, Fastenau PS, Perkins SM, Egelhoff JC, Kalnin A, Dunn DW, Austin JK. The association of MRI findings and neuropsychological functioning after the first recognized seizure. Epilepsia 2007;48:1067-74. [PubMed]
- Kassiri J, Snyder TJ, Bhargava R, Wheatley BM, Sinclair DB. Cortical tubers, cognition, and epilepsy in tuberous sclerosis. Pediatr Neurol 2011;44:328-32. [PubMed]
- Tosun D, Caplan R, Siddarth P, Seidenberg M, Gurbani S, Toga AW, Hermann B. Intelligence and cortical thickness in children with complex partial seizures. Neuroimage 2011;57:337-45. [PubMed]
- Caplan R, Levitt J, Siddarth P, Taylor J, Daley M, Wu KN, Gurbani S, Shields WD, Sankar R. Thought disorder and frontotemporal volumes in pediatric epilepsy. Epilepsy Behav 2008;13:593-9. [PubMed]
- Hermann B, Jones J, Sheth R, Dow C, Koehn M, Seidenberg M. Children with new-onset epilepsy: neuropsychological status and brain structure. Brain 2006;129:2609-19. [PubMed]
- Lawson JA, Vogrin S, Bleasel AF, Cook MJ, Burns L, McAnally L, Pereira J, Bye AM. Predictors of hippocampal, cerebral, and cerebellar volume reduction in childhood epilepsy. Epilepsia 2000;41:1540-5. [PubMed]
- Caplan R, Levitt J, Siddarth P, Wu KN, Gurbani S, Sankar R, Shields WD. Frontal and temporal volumes in Childhood Absence Epilepsy. Epilepsia 2009;50:2466-72. [PubMed]
- Caplan R, Levitt J, Siddarth P, Wu KN, Gurbani S, Shields WD, Sankar R. Language and brain volumes in children with epilepsy. Epilepsy Behav 2010;17:402-7. [PubMed]
- Lin JJ, Riley JD, Hsu DA, Stafstrom CE, Dabbs K, Becker T, Seidenberg M, Hermann BP. Striatal hypertrophy and its cognitive effects in new-onset benign epilepsy with centrotemporal spikes. Epilepsia 2012;53:677-85. [PubMed]
- Pulsipher DT, Seidenberg M, Guidotti L, Tuchscherer VN, Morton J, Sheth RD, Hermann B. Thalamofrontal circuitry and executive dysfunction in recent-onset juvenile myoclonic epilepsy. Epilepsia 2009;50:1210-9. [PubMed]
- Widjaja E, Skocic J, Go C, Snead OC, Mabbott D, Smith ML. Abnormal white matter correlates with neuropsychological impairment in children with localization-related epilepsy. Epilepsia 2013;54:1065-73. [PubMed]
- Braakman HM, Vaessen MJ, Jansen JF, Debeij-van Hall MH, de Louw A, Hofman PA, Vles JS, Aldenkamp AP, Backes WH. Pediatric frontal lobe epilepsy: white matter abnormalities and cognitive impairment. Acta Neurol Scand 2014;129:252-62. [PubMed]
- Bonilha L, Tabesh A, Dabbs K, Hsu DA, Stafstrom CE, Hermann BP, Lin JJ. Neurodevelopmental alterations of large-scale structural networks in children with new-onset epilepsy. Hum Brain Mapp 2014;35:3661-72. [PubMed]
- Vaessen MJ, Braakman HM, Heerink JS, Jansen JF, Debeij-van Hall MH, Hofman PA, Aldenkamp AP, Backes WH. Abnormal modular organization of functional networks in cognitively impaired children with frontal lobe epilepsy. Cereb Cortex 2013;23:1997-2006. [PubMed]
- Datta AN, Oser N, Bauder F, Maier O, Martin F, Ramelli GP, Steinlin M, Weber P, Penner IK. Cognitive impairment and cortical reorganization in children with benign epilepsy with centrotemporal spikes. Epilepsia 2013;54:487-94. [PubMed]
- Lange N, Froimowitz MP, Bigler ED, Lainhart JE; Brain Development Cooperative Group. Associations between IQ, total and regional brain volumes, and demography in a large normative sample of healthy children and adolescents. Dev Neuropsychol 2010;35:296-317. [PubMed]
- Booth T, Bastin ME, Penke L, Maniega SM, Murray C, Royle NA, Gow AJ, Corley J, Henderson RD, Hernández Mdel C, Starr JM, Wardlaw JM, Deary IJ. Brain white matter tract integrity and cognitive abilities in community-dwelling older people: the Lothian Birth Cohort, 1936. Neuropsychology 2013;27:595-607. [PubMed]
- Li Y, Liu Y, Li J, Qin W, Li K, Yu C, Jiang T. Brain anatomical network and intelligence. PLoS Comput Biol 2009;5:e1000395. [PubMed]
- Fischer FU, Wolf D, Scheurich A, Fellgiebel A. Association of structural global brain network properties with intelligence in normal aging. PLoS One 2014;9:e86258. [PubMed]
- Kanemura H, Sano F, Tando T, Sugita K, Aihara M. Repeated seizures induce prefrontal growth disturbance in frontal lobe epilepsy. Brain Dev 2012;34:175-80. [PubMed]
- Kanemura H, Hata S, Aoyagi K, Sugita K, Aihara M. Serial changes of prefrontal lobe growth in the patients with benign childhood epilepsy with centrotemporal spikes presenting with cognitive impairments/behavioral problems. Brain Dev 2011;33:106-13. [PubMed]
- Dabbs K, Jones JE, Jackson DC, Seidenberg M, Hermann BP. Patterns of cortical thickness and the Child Behavior Checklist in childhood epilepsy. Epilepsy Behav 2013;29:198-204. [PubMed]
- Richardson EJ, Griffith HR, Martin RC, Paige AL, Stewart CC, Jones J, Hermann BP, Seidenberg M. Structural and functional neuroimaging correlates of depression in temporal lobe epilepsy. Epilepsy Behav 2007;10:242-9. [PubMed]
- Daley M, Siddarth P, Levitt J, Gurbani S, Shields WD, Sankar R, Toga A, Caplan R. Amygdala volume and psychopathology in childhood complex partial seizures. Epilepsy Behav 2008;13:212-7. [PubMed]
- Rosso IM, Cintron CM, Steingard RJ, Renshaw PF, Young AD, Yurgelun-Todd DA. Amygdala and hippocampus volumes in pediatric major depression. Biol Psychiatry 2005;57:21-6. [PubMed]
- Lin JJ, Siddarth P, Riley JD, Gurbani SG, Ly R, Yee VW, Levitt JG, Toga AW, Caplan R. Neurobehavioral comorbidities of pediatric epilepsies are linked to thalamic structural abnormalities. Epilepsia 2013;54:2116-24. [PubMed]