A stubborn anemia caused by ectopic pancreas bleeding in the jejunum revealed by capsule endoscopy
Introduction
Ectopic pancreas, which can involve many parts of the intestinal tract, is a disease showing histological structures of normal pancreatic tissues but without any anatomical relation or direct connections by blood vessel to the pancreas. It is typically identified in the stomach and duodenum by gastroscopy, but it is rarely seen in the small intestine. To the best of our knowledge, there were few cases that have been detected by capsule endoscopy. Herein, we described an uncommon case of a stubborn anemia caused by ectopic pancreas bleeding in the jejunum, which was identified by capsule endoscopy.
Case presentation
A 70-year-old woman was admitted to our hospital because of anemia of undetermined origin, and gastrointestinal tumor was suspected. The patient did not report significant abdominal pain, distension, hematemesis, melena or significant weight loss.
On admission, she appeared stable on vital signs. The abdomen was soft and there was no tenderness and distension. Laboratory findings were otherwise normal except low serum hemoglobin (63 g/L) and ferritin levels (3.9 ng/mL). A full set of hemolytic anemia test and initial fecal occult blood test were unremarkable. Other laboratory tests such as biochemical assay, tumor markers and thyroid function were normal.
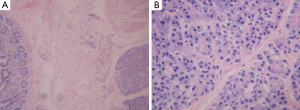
Spiral computed tomography (CT) enterography was not performed because the patient was allergic to varieties of drugs, including penicillin, streptomycin, sulfonamides and aminopyrine. Conventional proton pump inhibitor (PPI) and oral chalybeate was initiated. One week later, the repeated blood routine test showed that the hemoglobin level had dropped to 56 g/L, and the fecal occult blood test was positive [obscure bleeding (OB) ++++]. Such progressive decline of hemoglobin was thought to be caused by the existence of the active bleeding in digestive tract. Therefore, gastroscopy and colonoscopy were performed but failed to identify obvious bleeding lesions. Then we performed capsule endoscopy to evaluate the obscure gastrointestinal bleeding, which revealed a ridgy and centrally umbilicated lesion in the upper jejunum with active bleeding (Figures 1,2). The patient was not suggested to take operation immediately because of the low hemoglobin level. Laparotomy was performed after stabilization of hemoglobin. During the operation, a mass of 3×3 centimeters in diameter was found in the upper segment of jejunum. The histological examination revealed jejunal ectopic pancreas tissues involving the full-thickness of her jejunum.
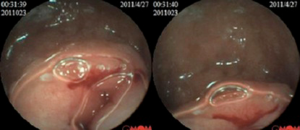
The patient recovered well postoperatively, and the hemoglobin rise to 91 g/L in 2 weeks. The red blood cell count was completely normal and the repeated stool OB test was negative in 4 months after discharge. She reported no discomfort during 1-year follow-up.
Discussion
Ectopic pancreas has histological structures of normal pancreatic tissue, but it has no anatomical relation with normal pancreas, and there were no direct connections by blood vessel between them. The histological features have been described in the literature (1), although it is rarely seen in the clinic. It is accidentally found in autopsy, operation or gastroscopy for other reasons in the past decades. More recently, a few cases detected by capsule endoscopy have been reported (2). The incidence in autopsies ranges 0.55-13.7%, being more common at the age of 30-50 years with a male predominance (with a male to female ratio of 3:1) (3,4).
The occurrence of ectopic pancreas is related to abnormal embryonic development (1). Several theories have been proposed to explain the pathogenesis and occurrence of ectopic pancreas. The normal pancreas is derived from several evaginations originating from the wall of the primitive duodenum. One theory implicates that during embryogenesis, if one or more evaginations remain in the wall of the bowel, it may be carried away from the remainder of the gland by the developing gastrointestinal tract and may give rise to ectopic pancreas. The other theory proposes pancreatic metaplasia of endodermal tissues that end up in the submucosa during embryonic life (5).
Most cases of ectopic pancreas are located in the upper gastrointestinal tract, and more than 90% of the cases are found in the stomach, duodenum, jejunum and Meckel’s diverticulum (4-7). Unusual locations include the gallbladder, bile ducts, splenic hilum, umbilicus, fallopian tubes, mediastinum, oesophagus, colon, omentum, lung, mediastinum, lymph nodes and retroperitoneum (4,6,8-13).
Ectopic pancreas is mostly located in mucosa and submucosa, following by muscular layer and serous layer. Only few involve the full thickness, such as lesions located in the stomach and duodenal bulb. Endoscopic ultrasonography (EUS) can help to determine in which layer it is located. Typical EUS features of aberrant pancreas are indistinct margin, heterogeneous appearance (mainly hypoechoic accompanied by scattered small hypoechoic areas), and location within either the third and fourth layers or only in the third layer. The lesion is commonly accompanied by an anechoic area and fourth-layer thickening. These features correlate closely with the histologic findings and are potentially useful in the preoperative diagnosis of aberrant pancreas (14,15).
Most cases of ectopic pancreas are asymptomatic, but symptoms may occur because of the hormones and enzymes secreted by the ectopic pancreatic tissue (16). Usually the patients with ectopic pancreas present nonspecific clinical symptoms, such as gastrointestinal bleeding, upper abdominal pain, incompletely pyloric or duodenal obstruction (17). Therefore, it is easy to be misdiagnosed as peptic ulcer, intestinal tumors, biliary tract disease, etc.
The asymptomatic ectopic pancreas can be treated just with Regular follow-up, while most experts agree that the symptomatic one should be treated aggressively. The treatments for ectopic pancreas include endoscopy and surgery. The type of the procedure should be performed according to the type and size of the pancreatic tissue (18). It is reported that the ectopic pancreas originated from the mucosa or submucosa can be done with mucosal resection of transparent cap under endoscope (7).
In our case, conventional gastroscopy and colonoscopy failed to find the cause of the bleeding, while capsule endoscopy had successfully detected the bleeding lesion and its location. Diagnosis of ectopic pancreas is extremely difficult during preoperative examination in patients, especially without capsule endoscopy (19).
Conclusions
In conclusion, capsule endoscopy played an important role in our case. Endoscopists should pay more attention to the ectopic pancreas as a rare differential diagnosis for intestinal lesion, in order to decrease the rate of misdiagnosis and missed diagnosis.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Consents: Written informed consent was obtained from the patient for publication of this case report. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- Christodoulidis G, Zacharoulis D, Barbanis S, Katsogridakis E, Hatzitheofilou K. Heterotopic pancreas in the stomach: a case report and literature review. World J Gastroenterol 2007;13:6098-100. [PubMed]
- Chen HL, Lin SC, Chang WH, Yang TL, Chen YJ. Identification of ectopic pancreas in the ileum by capsule endoscopy. J Formos Med Assoc 2007;106:240-3. [PubMed]
- Mulholland KC, Wallace WD, Epanomeritakis E, Hall SR. Pseudocyst formation in gastric ectopic pancreas. JOP 2004;5:498-501. [PubMed]
- Lai EC, Tompkins RK. Heterotopic pancreas. Review of a 26 year experience. Am J Surg 1986;151:697-700. [PubMed]
- Chandan VS, Wang W. Pancreatic heterotopia in the gastric antrum. Arch Pathol Lab Med 2004;128:111-2. [PubMed]
- Mortelé KJ, Rocha TC, Streeter JL, Taylor AJ. Multimodality imaging of pancreatic and biliary congenital anomalies. Radiographics 2006;26:715-31. [PubMed]
- Karahan OI, Kahriman G, Soyuer I, Artiş T, Comu NB. MR cholangiopancreatography findings of heterotopic pancreatic tissue in the distal common bile duct. Diagn Interv Radiol 2006;12:180-2. [PubMed]
- Silva AC, Charles JC, Kimery BD, Wood JP, Liu PT. MR Cholangiopancreatography in the detection of symptomatic ectopic pancreatitis in the small-bowel mesentery. AJR Am J Roentgenol 2006;187:W195-7. [PubMed]
- Marchevsky AM. Lung tumors derived from ectopic tissues. Semin Diagn Pathol 1995;12:172-84. [PubMed]
- Wang W, Li K, Qin W, Sun H, Zhao C. Ectopic pancreas in mediastinum: report of 2 cases and review of the literature. J Thorac Imaging 2007;22:256-8. [PubMed]
- Murayama H, Kikuchi M, Imai T, Yamamoto Y, Iwata Y. A case of heterotopic pancreas in lymph node. Virchows Arch A Pathol Anat Histol 1978;377:175-9. [PubMed]
- Wang C, Kuo Y, Yeung K, Wu C, Liu G. CT appearance of ectopic pancreas: a case report. Abdom Imaging 1998;23:332-3. [PubMed]
- Lin LH, Ko SF, Huang CC, Ng SH, Lin JW, Sheen-Chen SM. Retroperitoneal ectopic pancreas: imaging findings. Br J Radiol 2009;82:e253-5. [PubMed]
- Margolin DJ. Endoscopy-assisted laparoscopic resection of gastric heterotopic pancreas. Am Surg 2008;74:829-31. [PubMed]
- Matsushita M, Hajiro K, Okazaki K, Takakuwa H. Gastric aberrant pancreas: EUS analysis in comparison with the histology. Gastrointest Endosc 1999;49:493-7. [PubMed]
- Ormarsson OT, Gudmundsdottir I, Mårvik R. Diagnosis and treatment of gastric heterotopic pancreas. World J Surg 2006;30:1682-9. [PubMed]
- Lee MJ, Chang JH, Maeng IH, Park JY, Im YS, Kim TH, Han SW. Lee do S. Ectopic pancreas bleeding in the jejunum revealed by capsule endoscopy. Clin Endosc 2012;45:194-7. [PubMed]
- Chou SJ, Chou YW, Jan HC, Chen VT, Chen TH. Ectopic pancreas in the ampulla of vater with obstructive jaundice. A case report and review of literature. Dig Surg 2006;23:262-4. [PubMed]
- Fukino N, Oida T, Mimatsu K, Kida K, Kawasaki A, Kuboi Y, Kano H. Diffuse Peritonitis due to Perforated Gastric Ectopic Pancreas. Case Rep Gastroenterol 2012;6:689-94. [PubMed]


