Quantitative evaluation of SOCS-induced optical clearing efficiency of skull
Introduction
Advanced optical imaging methods combined with sophisticated labeling technology have shown a potential in visualization of both cortical structural and functional architecture with high spatial-temporal resolution (1-3). The optical imaging of neurons, gliocyte and vasculature in vivo does help to understand the cellular mechanisms underlying circuit plasticity, the process of the regulation of microcirculation and the immune response in cortex (4-8). However, the turbid skull above the cortex severely restricted the application of optical imaging technologies in brain research. In order to overcome the strong scattering of skull, several chronic cranial windows were proposed based on craniotomy (9-13), i.e., removed skull (9), open-skull window (10), thinned-skull cranial window (11), as well as polished and reinforced thinned skull window (12). However, these techniques are still not satisfactory. Surgical operations of removing skull will be unavoidable to change the normal physiological environment of the cortex, which induced cortical injury may lead to severe inflammatory reaction, and the activation of microglia to higher spine turnover rates (2,13). Thinned-skull window provides an invasive approach for in vivo studying structural and functional changes of neuron under normal and pathological conditions (11), but the imaging area is relative small and the image is easily blurred due to operator and thickness of the thinned skull (14). For polished and reinforced thinned skull window, the process is very complicated and the window is fragile and easy-polluted (12).
The tissue optical clearing technique is highly promising for reducing the turbid properties of skull. Recently, Zhu’s group invented an innovative skull optical clearing solution (SOCS). With this technique, the skull can be transparent within 25 minutes, and the high scattering restriction of the skull for cortical imaging in vivo could be overcome, and the cortical blood flow with high resolution were obtained (14-16).
Up till now, the efficiency of this method has not been evaluated comprehensively, so the character of the transparent skull window based on SOCS should be investigated quantitatively. For manipulation of cells in the cortex such as optogenetics and photo-induced disease model (17-19), it is deserved that the laser beam should be small enough when it reached the target area in cortex. Due to the scattering properties of skull, the laser beam will diffuse to a big spot, which will make it impossible to manipulate precisely. In addition, for observation of cells in the cortex, the energy densities will significantly decrease due to the attenuation and distortion of skull which will degrade the performance of cortical imaging (14,15). Therefore, whether the SOCS can restrain the divergence of light, increase the transmittance and decrease the scattering properties should be evaluated quantitatively.
The purpose of this study is to quantitatively evaluate the performance of SOCS systematically. Here, the improvement of divergence of beam spot and resolution were investigated before and after the treatment of SOCS on skull. Transmittance combined with Monte Carlo simulation was used to evaluate the changes in scattering coefficient after applying SOCS.
Materials and methods
Animals and agents
Adult Balb/c mice (male, 2 months, 22±3 g) were used in this study. SOCS used in our experiments consisted of various chemical biocompatible agents, which mainly contains laurinol, weak alkaline substances, EDTA, dimethyl sulfoxide, sorbitol, and glucose.
Mice were anesthetized with a cocktail of 2% chloralose and 10% urethane (8 mL/kg) via intraperitoneal injection and spinal dislocation to kill the mice. Then, the skull was taken out. Fascia as well as residual blood on skull should be clean with phosphate buffer saline (PBS) instantly. After that, the fresh skull was immersed in PBS and kept at 4 °C.
Measurement of laser beam spot and total transmittance
BC106-VIS beam profiler (Throlabs, USA), with 40 dB attenuation and MLL-III473 20 mW light source (Changchun New Industries, China) were applied in this experiment. Laser beam illuminated the sample, and the beam spot would be imaged by CCD closed to the sample, almost touching. The position of sample was moved slightly, and repeated measurements could be obtained. Next, the skull sample was treated with the optical clearing agent. And 25 min later, the sample experiment was repeated. In comparison, the original beam spot were recorded by using a neutral optical attenuator instead of skull.
In this study, the signal was smooth filtered first, and Gaussian function was fitted to calculate the size of laser beam as Eq. [1] (20),

Here I0 is maximum light intensity at the center of spot, I is light intensity in the distance of r to the center, and ω represents the size of laser beam spot.
Measuring collimation transmittance of skull
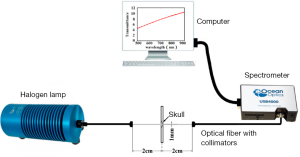
An optical fiber spectrometer (USB 4000, Ocean Optics, USA) was used to measure the transmittance of the mice skull. Figure 1 shows the schematic of measurement system. A light source (HL-2000-CAL) coupled with an optical fiber (QP600-2-VIS-NIR) was used to illuminate on the region of interest of the skull, and another same optical fiber coupled with spectrometer system was used to collect the transmission light in the spectral range of 400-900 nm. Here, two collimating lenses were coupled with the two optical fibers, respectively, so as to measure the collimated transmittance. The core diameter of the optical fiber is 600±10 µm.
The fresh and SOCS treated skull samples (6×6 mm2) obtained from the mice were put in the self-made black sample platform, which was with a pair of symmetric pinholes (φ=1 mm) on it (21). To obtain the collimation transmittance, the sample was in the middle of two collimation lens, and the distance between the two collimation lens was 4 cm. By applying this equipment, the transmittance spectrums of skull can be obtained before and after applying SOCS, respectively. And transmittance ratio (T) was calculated according to the following equation.

In the equation above, Ssample, Sempty and Sdark are on behalf of light intensity spectrum in the state of placing sample, removing sample and dark noise, respectively.
Collimation transmittance for calculating scattering coefficient based on Monte Carlo simulation
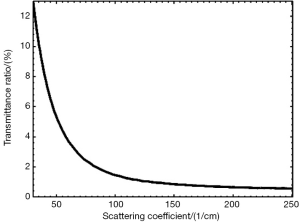
Monte Carlo simulation is a golden standard for simulating light transport and distribution in biological tissue (22-25). In order to obtain the scattering coefficient of skull, the relation between coefficient and collimation transmittance was established by using Monte Carlo simulation. Here, the skull was assumed as a uniform media, and the parameters of skull were set as follows, i.e., the absorption coefficient, mean refractive index and thickness of skull were set as follows, µa=0.5 cm–1, n=1.555, d=300 µm, g=0.911-0.958, respectively (26), and the scattering coefficient, µs=30-250 cm–1 due to optical clearing . The above parameters were thought to be unchangeable except µs. Therefore, it would obtain the number of transmittance photons in the area of detect probe by using Monte Carlo simulation, which would change with scattering coefficient of skull. Further, we could deduce the coefficient based on the measurement of collimation transmittance of skull.
Results
SOCS induced decrease in size of laser beam spot
Laser beam profiler is a common technique for evaluate quality of laser beam. If the laser beam passes through a turbid media, the laser profiler will be changed. Therefore, the laser beam profiler can be used to evaluate the scattering characteristics of media. Here, this method was adopted to quantitatively illustrate the efficiency of skull optical clearing. Figure 2A-C show the typical laser beam profiler through neutral optical attenuator, intact skull, and treated skull with SOCS, Figure 2D-F are the corresponding normalized optical intensity distribution alone the radial direct. Figure 2G shows the mean bandwidths of Gaussian for the three cases, and each value are from nine samples.
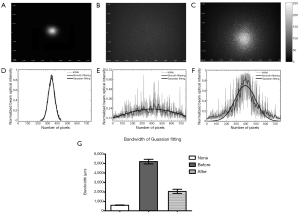
From the images showed above, it could be found that the laser spot is relative small through neutral optical attenuator. But it became fuzzy and lost the shape due to the diffusion of photons for intact skull. After the treatment of SOCS, photons were gathered and formed an evident circle. For the laser without passing by the skull, the diameter is 600±12 µm; before treated with SOCS, the laser beam was 5,192±250 µm. However, it reduced to 2,052±225 µm after skull optical clearing. The diameter of spot is significant reduced after the treatment.
SOCS induced decrease in scattering coefficient of skull
It is well known that tissue optical clearing can decrease the scattering coefficient. With the above set parameters, the collimation transmittance can be obtained with Monte Carlo simulation. Figure 3 shows the relations between transmittance and µs. The results show the transmittance decrease sharply with increasing scattering coefficient of media when the scattering coefficient is less than 100 cm–1. For stronger scattering media, there is hardly decrease in transmittance when scattering coefficient continue increases.
Figure 4A shows the measured transmittance in the wavelength range of 500-900 nm before or after treatment of SOCS on skull, which increases as the wavelength extending. For the intact skull, the transmittance of skull is relative low at 0.5% to 2%. After applying SOCS, the collimation transmittance raise with an overall upward trend from 5% to 12% in the same range of wavelength. Comparing two groups of data, it can be found that the SOCS made the transmittance of skull increase by 5 to 10 times.
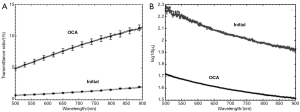
Comparing the measurements of collimation transmittance with the calculated results by Monte Carlo simulation shown in Figure 3, the scattering coefficient of skull with the wavelength (500-900 nm) in both cases was quantitatively calculated. In order to highlight how much the scattering coefficients of skull changed after applying SOCS, µs was converting to log10(µs). We could find that before applying SOCS, the scattering coefficients fluctuated with an overall downward trend from 185 to 85 cm–1 in the range from 500 to 900 nm. And after treated with SOCS, the scattering coefficients went down from 50 to 30 cm–1 in the range from 500 to 900 nm. Comparing the data, we could know that SOCS was able to make the scattering coefficients of skull reduce by 3 to 4 times.
Discussion
In this work, the efficiency of skull optical clearing was evaluated quantitatively. The results showed that the solutions can significant decrease the scattering coefficient of skull and keep some collimation of laser beam. It would make tremendous contributions to optical manipulation and imaging on cortex. For example, in the manipulation of cells in the cortex, the laser collimation will be severe destroyed by the high scattering properties of skull due to the attenuation and distortion introduced by skull. To realize the single cell manipulation and control, thinned skull or open skull windows were used, but those models based on surgical operations are not satisfactory (14,15). While, the SOCS could retain the shape and collimation of laser beam, which is hopeful to realize precision manipulation and control for optogenetics manipulation. In the establishment of animal models with diseases, the effect of skull is also a critical factor (12). For instance, an optically mediated occlusion to a single penetrating vessel is usually introduced by focal illumination of the intravenously injected photosensitizer with laser light, which led to a localized clot. But due to the high scattering properties, photo-induced mini-strokes via photosensitizers are hardly completed through the intact skull (12,19). The laser beam diffuse to a big one and the energy densities will significant decrease which will be not large enough to produce a clot in cortical vessels. Increasing the power of laser simply will be result in cortical injury and extensive mini-strokes. The SOCS could significant decrease the scattering coefficient of skull which will certainly increase the energy densities. It could overcome the technological challenges and difficulties by applying the SOCS in theory. Further, more work should be done in the future for the situation of in vivo is not exactly the same as in vitro.
Conclusions
In this study, the efficiency of skull optical clearing has been evaluated quantitatively, from the divergence of beam spot, the collimation transmittance to the scattering coefficient of skull. The results showed that the measured beam bandwidth reduced from 5.2±0.3 to 2.0±0.2 mm; and the scattering coefficient decreased nearly three folds after the treatment of SOCS on skull. This research provides important reference for performing cortical optical imaging or manipulation with high temporal-spatial resolution based on skull optical clearing technique.
Acknowledgements
This study was supported by the National Nature Science Foundation of China (Grant Nos. 81171376, 91232710, 812111313), the Science Fund for Creative Research Group (Grant No. 61121004), and the Research Fund for the Doctoral Program of Higher Education of China (Grant No. 20110142110073).
Disclosure: The authors declare no conflict of interest.
References
- Chen X, Leischner U, Rochefort NL, Nelken I, Konnerth A. Functional mapping of single spines in cortical neurons in vivo. Nature 2011;475:501-5. [PubMed]
- Kerr JN, Denk W. Imaging in vivo: watching the brain in action. Nat Rev Neurosci 2008;9:195-205. [PubMed]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 2007;8:700-11. [PubMed]
- Lai CS, Franke TF, Gan WB. Opposite effects of fear conditioning and extinction on dendritic spine remodelling. Nature 2012;483:87-91. [PubMed]
- Harvey CD, Svoboda K. Locally dynamic synaptic learning rules in pyramidal neuron dendrites. Nature 2007;450:1195-200. [PubMed]
- Drew PJ, Shih AY, Kleinfeld D. Fluctuating and sensory-induced vasodynamics in rodent cortex extend arteriole capacity. Proc Natl Acad Sci U S A 2011;108:8473-8. [PubMed]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 2005;308:1314-8. [PubMed]
- Azhan A, Wong FY. Challenges in understanding the impact of blood pressure management on cerebral oxygenation in the preterm brain. Front Physiol 2012;3:471. [PubMed]
- Daly SM, Leahy MJ. ‘Go with the flow’: a review of methods and advancements in blood flow imaging. J Biophotonics 2013;6:217-55. [PubMed]
- Holtmaat A, Bonhoeffer T, Chow DK, Chuckowree J, De Paola V, Hofer SB, Hübener M, Keck T, Knott G, Lee WC, Mostany R, Mrsic-Flogel TD, Nedivi E, Portera-Cailliau C, Svoboda K, Trachtenberg JT, Wilbrecht L. Long-term, high-resolution imaging in the mouse neocortex through a chronic cranial window. Nat Protoc 2009;4:1128-44. [PubMed]
- Yang G, Pan F, Parkhurst CN, Grutzendler J, Gan WB. Thinned-skull cranial window technique for long-term imaging of the cortex in live mice. Nat Protoc 2010;5:201-8. [PubMed]
- Drew PJ, Shih AY, Driscoll JD, Knutsen PM, Blinder P, Davalos D, Akassoglou K, Tsai PS, Kleinfeld D. Chronic optical access through a polished and reinforced thinned skull. Nat Methods 2010;7:981-4. [PubMed]
- Xu HT, Pan F, Yang G, Gan WB. Choice of cranial window type for in vivo imaging affects dendritic spine turnover in the cortex. Nat Neurosci 2007;10:549-51. [PubMed]
- Zhu D, Larin KV, Luo Q, Tuchin VV. Recent progress in tissue optical clearing. Laser Photon Rev 2013;7:732-57. [PubMed]
- Wang J, Zhang Y, Li P, Luo Q, Zhu D. Review: Tissue Optical Clearing Window for Blood Flow Monitoring Using Laser Speckle. IEEE Journal of Selected of Topics in Quantum Electronics 2014;20:6801112.
- Wang J, Zhang Y, Xu TH, Luo QM, Zhu D. An innovative transparent cranial window based on skull optical clearing. Laser Phys Lett 2012;9:469-73.
- Wilson NR, Runyan CA, Wang FL, Sur M. Division and subtraction by distinct cortical inhibitory networks in vivo. Nature 2012;488:343-8. [PubMed]
- Kravitz AV, Kreitzer AC. Optogenetic manipulation of neural circuitry in vivo. Curr Opin Neurobiol 2011;21:433-9. [PubMed]
- Blinder P, Shih AY, Rafie C, Kleinfeld D. Topological basis for the robust distribution of blood to rodent neocortex. Proc Natl Acad Sci U S A 2010;107:12670-5. [PubMed]
- Ioan MR, Gruia I, Ioan GV, Rusen L, Ioan P. Laser beam used to measure and highlight the transparency changes in gamma irradiated borosilicate glass. J Optoelectron Adv Mater 2014;16:167-74.
- Oliveira L, Carvalho MI, Nogueira E, Tuchin VV. Optical measurements of rat musle sample under treatment with ethylene glycol and glucose. J Innov Opt Health Sci 2013;6:1350012.
- Jiang C, He H, Li P, Luo Q. Graphics processing unit cluster accelerated Monte Carlo simulation of photon transport in multi-layered tissues. J Innov Opt Health Sci 2012;5:1250004.
- Zhu C, Liu Q. Validity of the semi-infinite tumor model in diffuse reflectance spectroscopy for epithelial cancer diagnosis: a Monte Carlo study. Opt Express 2011;19:17799-812. [PubMed]
- Luo B, He S. An improved Monte Carlo diffusion hybrid model for light reflectance by turbid media. Opt Express 2007;15:5905-18. [PubMed]
- Wang L, Jacques SL, Zheng L. MCML--Monte Carlo modeling of light transport in multi-layered tissues. Comput Methods Programs Biomed 1995;47:131-46. [PubMed]
- Vo-Dinh. eds. Biomedical photonics handbook. Boca Raton: CRC Press, 2003.