MRI assessment of cardiac tumours: part 1, multiparametric imaging protocols and spectrum of appearances of histologically benign lesions
Introduction
Cardiac magnetic resonance imaging (MRI) is considered the reference standard technique for characterization of a suspected cardiac mass. It provides an unrestricted field of view, high temporal resolution (30-50 ms) and non-invasive tissue characterization based on multi-parametric assessment of the chemical micro-environment (1). As such MRI is extremely helpful for diagnosis, assessment of the functional impact of a lesion, treatment planning and post treatment follow-up (2). We present a two-part review of the role of cardiac MRI in the assessment of cardiac tumours. Part one focuses on specific cardiac MRI techniques, protocol design and the appearance of histologically benign tumours. Part two covers histologically malignant tumours, and also reviews the MRI appearance of potential tumour mimics.
Primary cardiac tumours are extremely rare with an estimated lifetime incidence across all age groups of 0.0017-0.02% (3,4). Among the primary tumours approximately 75% are benign with more than half of these being myxomas (5). Histologically malignant tumours are predominantly sarcomatous in nature (6-8). By comparison, metastatic involvement of the heart is 100-500 times more frequent than primary tumours (9). Cardiac tumours are frequently asymptomatic and often discovered incidentally during evaluation of an unrelated problem or physical finding. Symptoms and signs depend on the size and location of the tumour, haemodynamic effects (chamber obstruction or embolization) and interference with cardiac conduction (heart blocks or cardiac arrhythmias). Benign primary tumours are often amenable to complete surgical resection with low mortality and morbidity and most patients have a good prognosis if surgery is successful (10). Conversely the prognosis for patients with malignant cardiac sarcomas is dismal with a median survival of only 6-12 months (11), although some may enjoy long-term survival with a radical multidisciplinary approach (12). Most malignant lesions cannot be completely resected but patients may gain symptomatic benefit from a debulking procedure.
Most cardiac masses are not amenable to percutaneous or catheter biopsy and imaging plays a crucial role in their evaluation. Transthoracic echocardiography (TTE) is usually the first line imaging technique but is often unable to provide complete evaluation due to its limited field of view and limited soft tissue resolution (13). Transesophageal echocardiography (TOE) improves image quality and provides more detailed assessment but also has limited tissue characterization abilities. Multi-detector computed tomography (MDCT) has an emerging role in the assessment of a cardiac mass due to its high spatial resolution (0.4-0.6 mm), short examination time (especially useful in patients with orthopnoea) and ability to definitively detect fat and calcification. Currently the main disadvantage of MDCT is a lack of inherent soft tissue contrast resolution (14,15). Cardiac MRI is considered to be the gold standard technique for assessing a suspected cardiac tumour owing to its unique ability to provide non-invasive tissue characterization in conjunction with high temporal resolution and lack of exposure to ionizing radiation (16,17). In day to day practice echocardiography, MDCT and MRI are frequently used complimentary to one another to provide as much information as possible concerning a suspected cardiac tumour.
Cardiac MRI sequences
A variety of MRI pulse sequences can be used to confirm the presence of a suspected cardiac mass and help assess its tissue composition and impact on adjacent structures including the valves and coronary arteries. MRI exploits differences in hydrogen proton density in conjunction with T1 and T2 relaxation properties of different tissues to help differentiation normal from abnormal and benign from malignant lesions. As malignant cells have a higher free intracellular water content and a greater degree of surrounding extracellular fluid due to interstitial oedema they usually have longer T1 and T2 relaxation times compared with benign pathology (15). Malignant tissue is also associated with a greater degree of neo-angiogensis and hence first pass contrast enhancement. Necrotic tumour tissue is associated with interstitial expansion and the accumulation and delayed washout of gadolinium based contrast agents (2).
Black blood prepared techniques
“Black-blood” prepared static MRI images are used to help localise a suspected cardiac or juxta cardiac mass and to non-invasively assess its tissue composition. Black blood images are usually acquired using a double-inversion recovery fast spin-echo sequence whereby the initial 180° inversion pulse is non slice selective and followed by a slice-selective 180° pulse (18). This causes blood flowing into the slice to undergo only the first non-selective 180° inversion pulse giving it a black-blood appearance. To achieve T1-weighted images the time to repeat (TR) is set at around 1,000 ms; T2-weighted images require a TR of around 2,000 ms which doubles the acquisition time. The addition of a third slice selective 180° inversion pulse (triple inversion recovery) can be used to give fat saturation which is helpful for characterisation of a fat containing lesion (19).
Bright blood prepared techniques
Cine steady-state free precession (SSFP) is an un-enhanced fast gradient echo technique which uses a short TR (2-3 msec) and segmented k-space filling. SSFP enables cine cardiac imaging at high temporal resolution and at the same time gives excellent contrast definition between the blood pool and myocardium. SSFP has utility for assessing the mobility and attachment point of a mass as well as its dynamic relationship and impact upon the valve structures. SSFP should not be used for assessment of tissue composition of a cardiac mass as its tissue weighting is dependent on both T1 and T2 effects (2,20).
First pass perfusion and delayed enhancement imaging
First pass rest perfusion imaging using a T1-weighted gradient echo sequence and infusion of gadolinium chelate followed by 10-15 minutes delayed enhancement imaging (using a T1-weighted inversion recovery sequence) assesses lesion vascularity. First pass enhancement is typical of highly vascular tumours such as haemangioma or angiosarcoma. The presence of late phase gadolinium enhancement (LGE) implies delayed washout from a lesion, usually as the result of extracellular space expansion or necrosis (21). LGE can be seen with both benign and malignant lesions. Benign tumours like fibroma usually display uniform LGE whereas tumours with a more heterogeneous composition like myxoma or angiosarcoma (containing a mixture of tumour tissue, necrosis and foci of haemorrhage) usually show patchy LGE (22).
MRI tumour protocol
At our institution cardiac MRI is performed on a 1.5 Tesla scanner (Ingenia, Philips Healthcare, Best, The Netherlands). A summary of our institutional imaging protocol for a suspected cardiac mass is presented in Table 1. Black blood images in both the axial and coronal planes are obtained with both T1-and T2-weighting and then followed by fat-suppressed triple inversion recovery images. SSFP sequences are acquired as a contiguous axial plane stack from the diaphragm to aortic arch as well as along the left ventricular short axis from base to cardiac apex. Additional 2-chamber, 4-chamber and left ventricular outflow tract plane SSFP cines are also acquired to provide optimal assessment of the valves. We then acquire a rest perfusion sequence which is planned through the centre of any mass detected in at least two planes followed by a short stack of LGE images in the same planes.
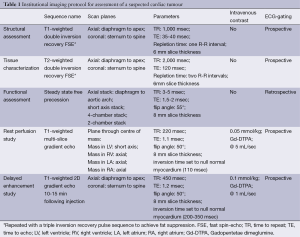
Full table
Benign primary tumors
The approximate frequency of benign primary cardiac tumours presenting in adulthood is presented in Table 2. Most benign tumours are intra-cavitary with a narrow attachment point. Those that arise within the peri- or myocardium are usually well defined, with a homogeneous appearance and narrow zone of transition (23).
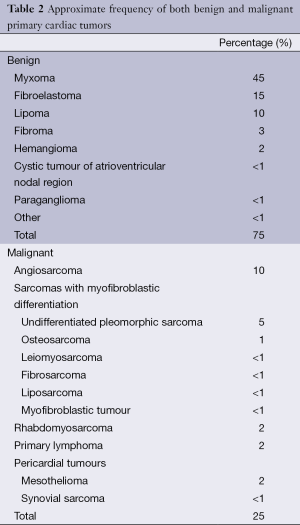
Full table
Myxoma
Myxoma is the most common primary cardiac neoplasm and is believed to originate from multipotent mesenchymal cell residues within the endocardium (24). Most arise from an area immediately adjacent to the fossa ovalis with a narrow pedicle and villous surface extensions which are friable and at risk of embolization (25). The vast majority develop within the left atrium and are solitary (26-28). Multiple myxomas are rare and usually seen in association with Carney complex which is an autosomal dominant syndrome characterized by multiple myxomas (including extracardiac myxomas), schwannomas and various endocrine tumours (29,30). Mean age at presentation is 50 years with a slight female predominance (31). Clinical symptoms are wide ranging and may relate to intracardiac obstruction (often mimicking mitral stenosis), systemic embolisation (stroke) or a systemic inflammatory response (arthralgia, fevers) (32-34). Prompt surgical resection of myxoma is advocated for definite diagnosis and to reduce the risk of complications (35-39). The risk of recurrence or development of new lesions is low (estimated at 2-5%) and annual TTE follow-up is recommended for a minimum of 4-year post resection (11).
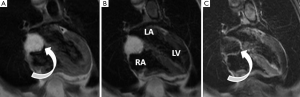
Typical MRI features are a well-defined spherical or ovoid mobile lobulated mass within either atria, sometimes prolapsing through the atrioventricular valve orifice in diastole. They usually appear hypointense to the blood pool on SSFP sequences (2). T1 and T2-weighted images usually show a heterogeneous appearance because of the combination of tumour tissue, necrotic elements and foci of haemorrhage and calcification (40-42) (Figure 1). First pass perfusion is usually absent and LGE is often multi-focal and patchy although may sometimes be homogeneous.
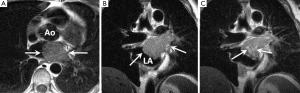
The main imaging differential for myxoma is thrombus. Key discrimating features which are usually reliable for distinguishing thrombus from myxoma are an absence of LGE and lack of atrioventricular valve prolapse (43).
Papillary fibroelastoma
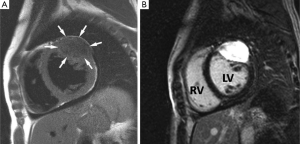
Fibroelastoma is the most common primary tumour of the cardiac valves although may arise from any endocardial surface (44). They are usually small lesions (<1.5 cm) and are composed of collagen and elastic tissue fibres lined by endothelium with a short pedicle (45). Commonest sites are the atrial side of the mitral valve and aortic surface of the aortic valve leaflets. The majority are asymptomatic and observed serendipitously at TTE performed for another reason but on occasion they may cause symptoms as a result of embolization of tumour fragments or surface thrombus into the systemic or pulmonary circulations (45). Aortic valve fibroelastomas can rarely be a cause of sudden death due to presumed acute obstruction of the coronary artery ostia (46). Prompt surgical resection is the treatment of choice, especially for left sided lesions (40).
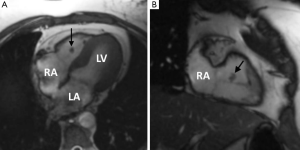
TOE is the modality of choice to detect these small highly mobile lesions and MRI is rarely required. When imaged with MRI features are those of a low signal well circumscribed mobile valve nodule on SSFP sequences, often with peri-lesional flow artifact (47-49) (Figure 2). T1 and T2 weighted images reflect their fibroelastic composition with uniform intermediate signal intensity similar to myocardium. Homogeneous LGE has been reported but in our experience LGE is commonly absent in keeping with a poorly vascularised lesion (50). The main imaging differential is a vegetation of infective endocarditis but distinction is generally straightforward on clinical grounds. On imaging vegetations are invariably associated with valve destruction/incompetence unlike a fibroelastoma with which valve function is usually preserved (51).
Lipoma
Lipomas are an encapsulated conglomerate of slow growing mature adipose tissue. They can occur anywhere in the heart but are most frequently seen within the atrial septum and epicardium where they grow into the pericardial space. Subendocardial lipomas are broad based lesions which protrude into the cardiac chambers and may cause obstructive and compressive effects but most probably remain asymptomatic and are discovered incidentally (52-54). There is a reported association with atrial arrhythmias and conduction abnormalities. Surgical resection is rarely needed except with very large and symptomatic lesions (40).
Lipomatous hypertrophy of the inter-atrial septum is hyperplasia of normal septal fat and also reflects a benign entity. The key distinguishing feature from lipoma is sparing of the mid-septal region (fossa ovalis) giving rise to their classical “dumbbell” appearance.
Lipomas and lipomatous hypertrophy have a highly variable appearance on echocardiography making confident distinction from other cardiac tumours like myxoma challenging (55). MRI appearances are typical owing to definitive characterization of fat signal which appears of uniformly high signal on both T1 and T2-weighted imaging and fully suppresses with fat saturation (Figure 3). Due to their avascular nature there is no first pass or LGE with these lesions (56).
Haemangioma
These are slow flow vascular malformations composed of endothelial lined venous channels with an intervening connective tissue stroma (56). There is no chamber predilection and they have been described in many locations including with the pericardial space. Most probably remain asymptomatic but large lesions are associated with exertional dyspnoea and may be considered for surgical resection.
On MRI they display isointense signal to myocardium on T1-weighted images due to slow flow and of diffuse high signal intensity on T2-weighted images. First pass and delayed enhancement is intense and prolonged and may appear heterogeneous depending upon the amount of fibrous tissue and any calcific foci (57).
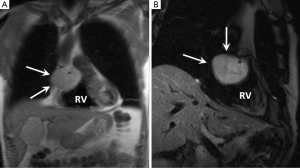
Fibroma
Fibromas are the most common paediatric cardiac tumour but they are occasionally seen in adults (58-60). Typically, they have an intramural location within the interventricular septum or left ventricular free wall (61). Fibromas have been associated with Polyposis syndromes (familial adenomatous polyposis) and Gorlin-Goltz syndrome (62,63). Histologically they are well-defined lesions composed of collagen and fibroblasts with some calcific foci which help differentiate them from rhabdomyomas. Heart failure is the most common clinical presentation due to obstruction of blood flow or interference with valvular function. Ventricular arrhythmias and sudden death have also been reported (63).
On MRI fibromas are usually sharply demarcated and of uniformly low signal intensity compared with adjacent myocardium on both T1 and T2-weighted imaging due to their dense fibrous composition (64). A lack of first pass enhancement is typical and LGE is commonly intense and uniform although peripheral and heterogenous LGE patterns have also been described (65,66) (Figure 4). The main imaging differential of a fibroma with little or no delayed enhancement is focal hypertrophic cardiomyopathy and distinction can be challenging. Careful review of the cine images is the best means of discrimination with a fibroma showing no regions of contractility which can usually be appreciated in most cases of “mass-like” hypertrophic cardiomyopathy (65).
Cardiac paraganglioma
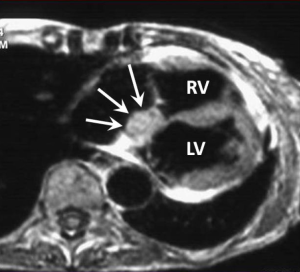
Paraganglioma is a very rare tumour which originates from neuroendocrine ganglia cells which tend to lie within the atrioventricular grooves and at the root of the great vessel origins (67). Up to 50% of these tumours secrete catecholamines which explains the typical clinical presentation of palpitations, flushing and headaches. Patients may also be hypertensive. Although histologically benign curative resection is often difficult due to extensive vascularity and often complex relationship with the adjacent coronary arteries.
On MRI these lesions usually range in size from 4-8 cm appearing well circumscribed. They are isointense to myocardium on T1- and of intensely high signal on T2-weighted images (“light bulb bright”) (Figure 5). First pass and LGE is usually intense and uniform (23).
Cystic tumour of the atrioventricular node
This is one of the rarest benign cardiac tumours with only a few cases reported in the literature. It is postulated to be an inclusion cyst of endodermal origin containing keratinous debris. Most reported lesions are small (<2 cm) and located at the base of the interatrial septum in the atrioventricular nodal region where they may cause interference with cardiac conduction leading to heart block and potentially lethal arrhythmias. Surgical resection is therefore advocated (68,69).
Described MRI features are those of a well-defined high T1 and high T2-signal nodule in the AV nodal region of the lower septum (Figure 6). Homogeneous LGE has been reported (70).
Conclusions
A comprehensive cardiac tumour imaging protocol has been described and the spectrum of benign primary tumour MRI appearances has been reviewed. Part 2 of this review will focus on primary malignant cardiac tumours, cardiac metastases as well as the MRI features of some potential tumour mimics.
Disclosure: The authors declare no conflict of interest.
References
- Kaminaga T, Takeshita T, Kimura I. Role of magnetic resonance imaging for evaluation of tumors in the cardiac region. Eur Radiol 2003;13 Suppl 4:L1-10.
- Sparrow PJ, Kurian JB, Jones TR, Sivananthan MU. MR imaging of cardiac tumors. Radiographics 2005;25:1255-76. [PubMed]
- Reynen K. Frequency of primary tumors of the heart. Am J Cardiol 1996;77:107. [PubMed]
- Centofanti P, Di Rosa E, Deorsola L, Dato GM, Patanè F, La Torre M, Barbato L, Verzini A, Fortunato G, di Summa M. Primary cardiac tumors: early and late results of surgical treatment in 91 patients. Ann Thorac Surg 1999;68:1236-41. [PubMed]
- Lam KY, Dickens P, Chan AC. Tumors of the heart. A 20-year experience with a review of 12,485 consecutive autopsies. Arch Pathol Lab Med 1993;117:1027-31. [PubMed]
- Molina JE, Edwards JE, Ward HB. Primary cardiac tumors: experience at the University of Minnesota. Thorac Cardiovasc Surg 1990;38 Suppl 2:183-91. [PubMed]
- Orlandi A, Ferlosio A, Roselli M, Chiariello L, Spagnoli LG. Cardiac sarcomas: an update. J Thorac Oncol 2010;5:1483-9. [PubMed]
- Simpson L, Kumar SK, Okuno SH, Schaff HV, Porrata LF, Buckner JC, Moynihan TJ. Malignant primary cardiac tumors: review of a single institution experience. Cancer 2008;112:2440-6. [PubMed]
- Chiles C, Woodard PK, Gutierrez FR, Link KM. Metastatic involvement of the heart and pericardium: CT and MR imaging. Radiographics 2001;21:439-49. [PubMed]
- Bakaeen FG, Reardon MJ, Coselli JS, Miller CC, Howell JF, Lawrie GM, Espada R, Ramchandani MK, Noon GP, Weilbaecher DG, DeBakey ME. Surgical outcome in 85 patients with primary cardiac tumors. Am J Surg 2003;186:641-7; discussion 647. [PubMed]
- Donsbeck AV, Ranchere D, Coindre JM, Le Gall F, Cordier JF, Loire R. Primary cardiac sarcomas: an immunohistochemical and grading study with long-term follow-up of 24 cases. Histopathology 1999;34:295-304. [PubMed]
- Shapira OM, Korach A, Izhar U, Koler T, Wald O, Ayman M, Erez E, Blackmon SH, Reardon MJ. Radical multidisciplinary approach to primary cardiac sarcomas. Eur J Cardiothorac Surg 2013;44:330-5; discussion 335-6. [PubMed]
- Sütsch G, Jenni R, von Segesser L, Schneider J. Heart tumors: incidence, distribution, diagnosis. Exemplified by 20,305 echocardiographies. Schweiz Med Wochenschr 1991;121:621-9. [PubMed]
- Roberts WT, Bax JJ, Davies LC. Cardiac CT and CT coronary angiography: technology and application. Heart 2008;94:781-92. [PubMed]
- Mitchell DG, Burk DL Jr, Vinitski S, Rifkin MD. The biophysical basis of tissue contrast in extracranial MR imaging. AJR Am J Roentgenol 1987;149:831-7. [PubMed]
- Bluemke DA, Achenbach S, Budoff M, Gerber TC, Gersh B, Hillis LD, Hundley WG, Manning WJ, Printz BF, Stuber M, Woodard PK. Noninvasive coronary artery imaging: magnetic resonance angiography and multidetector computed tomography angiography: a scientific statement from the american heart association committee on cardiovascular imaging and intervention of the council on cardiovascular radiology and intervention, and the councils on clinical cardiology and cardiovascular disease in the young. Circulation 2008;118:586-606. [PubMed]
- Pennell DJ, Sechtem UP, Higgins CB, Manning WJ, Pohost GM, Rademakers FE, van Rossum AC, Shaw LJ, Yucel EK; Society for Cardiovascular Magnetic Resonance; Working Group on Cardiovascular Magnetic Resonance of the European Society of Cardiology. Clinical indications for cardiovascular magnetic resonance (CMR): Consensus Panel report. Eur Heart J 2004;25:1940-65. [PubMed]
- Stehling MK, Holzknecht NG, Laub G, Böhm D, von Smekal A, Reiser M. Single-shot T1- and T2-weighted magnetic resonance imaging of the heart with black blood: preliminary experience. MAGMA 1996;4:231-40. [PubMed]
- Simonetti OP, Finn JP, White RD, Laub G, Henry DA. “Black blood” T2-weighted inversion-recovery MR imaging of the heart. Radiology 1996;199:49-57. [PubMed]
- Hansen MW, Merchant N. MRI of hypertrophic cardiomyopathy: part I, MRI appearances. AJR Am J Roentgenol 2007;189:1335-43. [PubMed]
- Kramer CM, Barkhausen J, Flamm SD, Kim RJ, Nagel E; Society for Cardiovascular Magnetic Resonance Board of Trustees Task Force on Standardized Protocols. Standardized cardiovascular magnetic resonance imaging (CMR) protocols, society for cardiovascular magnetic resonance: board of trustees task force on standardized protocols. J Cardiovasc Magn Reson 2008;10:35. [PubMed]
- Hoffmann U, Globits S, Schima W, Loewe C, Puig S, Oberhuber G, Frank H. Usefulness of magnetic resonance imaging of cardiac and paracardiac masses. Am J Cardiol 2003;92:890-5. [PubMed]
- Reynen K. Cardiac myxomas. N Engl J Med 1995;333:1610-7. [PubMed]
- Kono T, Koide N, Hama Y, Kitahara H, Nakano H, Suzuki J, Isobe M, Amano J. Expression of vascular endothelial growth factor and angiogenesis in cardiac myxoma: a study of fifteen patients. J Thorac Cardiovasc Surg 2000;119:101-7. [PubMed]
- Hoey ET, Mankad K, Puppala S, Gopalan D, Sivananthan MU. MRI and CT appearances of cardiac tumours in adults. Clin Radiol 2009;64:1214-30. [PubMed]
- Carney JA, Hruska LS, Beauchamp GD, Gordon H. Dominant inheritance of the complex of myxomas, spotty pigmentation, and endocrine overactivity. Mayo Clin Proc 1986;61:165-72. [PubMed]
- Pinede L, Duhaut P, Loire R. Clinical presentation of left atrial cardiac myxoma. A series of 112 consecutive cases. Medicine (Baltimore) 2001;80:159-72. [PubMed]
- Tazelaar HD, Locke TJ, McGregor CG. Pathology of surgically excised primary cardiac tumors. Mayo Clin Proc 1992;67:957-65. [PubMed]
- Carney JA, Gordon H, Carpenter PC, Shenoy BV, Go VL. The complex of myxomas, spotty pigmentation, and endocrine overactivity. Medicine (Baltimore) 1985;64:270-83. [PubMed]
- Casey M, Mah C, Merliss AD, Kirschner LS, Taymans SE, Denio AE, Korf B, Irvine AD, Hughes A, Carney JA, Stratakis CA, Basson CT. Identification of a novel genetic locus for familial cardiac myxomas and Carney complex. Circulation 1998;98:2560-6. [PubMed]
- Burke A, Virmani R. Tumors of the heart and great vessels In: Atlas of tumor pathology: fasc 16, ser 3. Washington, DC: Armed Forces Institute of Pathology, 1996:1-98.
- Markel ML, Waller BF, Armstrong WF. Cardiac myxoma. A review. Medicine (Baltimore) 1987;66:114-25. [PubMed]
- Eckhardt BP, Dommann-Scherrer CC, Stuckmann G, Zollikofer CL, Wentz KU. Giant cardiac myxoma with malignant transformed glandular structures. Eur Radiol 2003;13:2099-102. [PubMed]
- Todo T, Usui M, Nagashima K. Cerebral metastasis of malignant cardiac myxoma. Surg Neurol 1992;37:374-9. [PubMed]
- Keeling IM, Oberwalder P, Anelli-Monti M, Schuchlenz H, Demel U, Tilz GP, Rehak P, Rigler B. Cardiac myxomas: 24 years of experience in 49 patients. Eur J Cardiothorac Surg 2002;22:971-7. [PubMed]
- Selkane C, Amahzoune B, Chavanis N, Raisky O, Robin J, Ninet J, Obadia JF. Changing management of cardiac myxoma based on a series of 40 cases with long-term follow-up. Ann Thorac Surg 2003;76:1935-8. [PubMed]
- Cina SJ, Smialek JE, Burke AP, Virmani R, Hutchins GM. Primary cardiac tumors causing sudden death: a review of the literature. Am J Forensic Med Pathol 1996;17:271-81. [PubMed]
- Jelic J, Milicić D, Alfirević I, Anić D, Baudoin Z, Bulat C, Corić V, Dadić D, Husar J, Ivanćan V, Korda Z, Letica D, Predrijevac M, Ugljen R, Vućemilo I. Cardiac myxoma: diagnostic approach, surgical treatment and follow-up. A twenty years experience. J Cardiovasc Surg (Torino) 1996;37:113-7. [PubMed]
- Bakaeen FG, Reardon MJ, Coselli JS, Miller CC, Howell JF, Lawrie GM, Espada R, Ramchandani MK, Noon GP, Weilbaecher DG, DeBakey ME. Surgical outcome in 85 patients with primary cardiac tumors. Am J Surg 2003;186:641-7; discussion 647. [PubMed]
- Bruce CJ. Cardiac tumours: diagnosis and management. Heart 2011;97:151-60. [PubMed]
- Singh SD, Lansing AM. Familial cardiac myxoma--a comprehensive review of reported cases. J Ky Med Assoc 1996;94:96-104. [PubMed]
- Schvartzman PR, White RD. Imaging of cardiac and paracardiac masses. J Thorac Imaging 2000;15:265-73. [PubMed]
- Scheffel H, Baumueller S, Stolzmann P, Leschka S, Plass A, Alkadhi H, Schertler T. Atrial myxomas and thrombi: comparison of imaging features on CT. AJR Am J Roentgenol 2009;192:639-45. [PubMed]
- van Werkum MH, Swaans MJ, van Es HW, Rensing B, van Heesewijk JP. Case 190: papillary fibroelastoma of the pulmonary valve. Radiology 2013;266:680-4. [PubMed]
- Sun JP, Asher CR, Yang XS, Cheng GG, Scalia GM, Massed AG, Griffin BP, Ratliff NB, Stewart WJ, Thomas JD. Clinical and echocardiographic characteristics of papillary fibroelastomas: a retrospective and prospective study in 162 patients. Circulation 2001;103:2687-93. [PubMed]
- Wintersperger BJ, Becker CR, Gulbins H, Knez A, Bruening R, Heuck A, Reiser MF. Tumors of the cardiac valves: imaging findings in magnetic resonance imaging, electron beam computed tomography, and echocardiography. Eur Radiol 2000;10:443-9. [PubMed]
- Sengupta PP, Khandheria BK. Transoesophageal echocardiography. Heart 2005;91:541-7. [PubMed]
- Kondruweit M, Schmid M, Strecker T. Papillary fibroelastoma of the mitral valve: appearance in 64-slice spiral computed tomography, magnetic resonance imaging, and echocardiography. Eur Heart J 2008;29:831. [PubMed]
- Syed IS, Feng D, Harris SR, Martinez MW, Misselt AJ, Breen JF, Miller DV, Araoz PA. MR imaging of cardiac masses. Magn Reson Imaging Clin N Am 2008;16:137-64. [PubMed]
- Kelle S, Chiribiri A, Meyer R, Fleck E, Nagel E. Images in cardiovascular medicine. Papillary fibroelastoma of the tricuspid valve seen on magnetic resonance imaging. Circulation 2008;117:e190-1. [PubMed]
- Klarich KW, Enriquez-Sarano M, Gura GM, Edwards WD, Tajik AJ, Seward JB. Papillary fibroelastoma: echocardiographic characteristics for diagnosis and pathologic correlation. J Am Coll Cardiol 1997;30:784-90. [PubMed]
- Friedberg MK, Chang IL, Silverman NH, Ramamoorthy C, Chan FP. Images in cardiovascular medicine. Near sudden death from cardiac lipoma in an adolescent. Circulation 2006;113:e778-9. [PubMed]
- Puvaneswary M, Edwards JR, Bastian BC, Khatri SK. Pericardial lipoma: ultrasound, computed tomography and magnetic resonance imaging findings. Australas Radiol 2000;44:321-4. [PubMed]
- Lang-Lazdunski L, Oroudji M, Pansard Y, Vissuzaine C, Hvass U. Successful resection of giant intrapericardial lipoma. Ann Thorac Surg 1994;58:238-40; discussion 240-1. [PubMed]
- Restrepo CS, Largoza A, Lemos DF, Diethelm L, Koshy P, Castillo P, Gomez R, Moncada R, Pandit M. CT and MR imaging findings of benign cardiac tumors Curr Probl Diagn Radiol 2005;34:12-21. [PubMed]
- Grebenc ML, Rosado de Christenson ML, Burke AP, Green CE, Galvin JR. Primary cardiac and pericardial neoplasms: radiologic-pathologic correlation. Radiographics 2000;20:1073-103; quiz 1110-1, 1112.
- Oshima H, Hara M, Kono T, Shibamoto Y, Mishima A, Akita S. Cardiac hemangioma of the left atrial appendage: CT and MR findings. J Thorac Imaging 2003;18:204-6. [PubMed]
- ElBardissi AW, Dearani JA, Daly RC, Mullany CJ, Orszulak TA, Puga FJ, Schaff HV. Analysis of benign ventricular tumors: long-term outcome after resection. J Thorac Cardiovasc Surg 2008;135:1061-8. [PubMed]
- Valente M, Cocco P, Thiene G, Casula R, Poletti A, Milanesi O, Fasoli G, Livi U. Cardiac fibroma and heart transplantation. J Thorac Cardiovasc Surg 1993;106:1208-12. [PubMed]
- Burke AP, Rosado-de-Christenson M, Templeton PA, Virmani R. Cardiac fibroma: clinicopathologic correlates and surgical treatment. J Thorac Cardiovasc Surg 1994;108:862-70. [PubMed]
- Cho JM, Danielson GK, Puga FJ, Dearani JA, McGregor CG, Tazelaar HD, Hagler DJ. Surgical resection of ventricular cardiac fibromas: early and late results. Ann Thorac Surg 2003;76:1929-34. [PubMed]
- Yang HS, Arabia FA, Chaliki HP, De Petris G, Khandheria BK, Chandrasekaran K. Images in cardiovascular medicine. Left atrial fibroma in gardner syndrome: real-time 3-dimensional transesophageal echo imaging. Circulation 2008;118:e692-6. [PubMed]
- Herman TE, Siegel MJ, McAlister WH. Cardiac tumor in Gorlin syndrome. Nevoid basal cell carcinoma syndrome. Pediatr Radiol 1991;21:234-5. [PubMed]
- Brechtel K, Reddy GP, Higgins CB. Cardiac fibroma in an infant: magnetic resonance imaging characteristics. J Cardiovasc Magn Reson 1999;1:159-61. [PubMed]
- O’Donnell DH, Abbara S, Chaithiraphan V, Yared K, Killeen RP, Cury RC, Dodd JD. Cardiac tumors: optimal cardiac MR sequences and spectrum of imaging appearances. AJR Am J Roentgenol 2009;193:377-87. [PubMed]
- Kiaffas MG, Powell AJ, Geva T. Magnetic resonance imaging evaluation of cardiac tumor characteristics in infants and children. Am J Cardiol 2002;89:1229-33. [PubMed]
- Manger WM. An overview of pheochromocytoma: history, current concepts, vagaries, and diagnostic challenges. Ann N Y Acad Sci 2006;1073:1-20. [PubMed]
- Kaminishi Y, Watanabe Y, Nakata H, Shimokama T, Jikuya T. Cystic tumor of the atrioventricular nodal region. Jpn J Thorac Cardiovasc Surg 2002;50:37-9. [PubMed]
- Butany J, Nair V, Naseemuddin A, Nair GM, Catton C, Yau T. Cardiac tumours: diagnosis and management. Lancet Oncol 2005;6:219-28. [PubMed]
- Tran TT, Starnes V, Wang X, Getzen J, Ross BD. Cardiovascular magnetics resonance diagnosis of cystic tumor of the atrioventricular node. J Cardiovasc Magn Reson 2009;11:13. [PubMed]


