
Unusual uptakes on 18F-fluorocholine positron emission tomography/computed tomography (PET/CT): a retrospective study of 368 prostate cancer patients referred for a biochemical recurrence or an initial staging
Introduction
Prostate cancer (PCa) is the 2nd most common cancer in men (1), and the 5-year biochemical recurrence-free survival strongly depends on the histological grade and on the initial stage at the time of diagnosis (2,3). Approximately 15–40% of treated patients will experience local or distant recurrence during follow-up. Most of these recurrences will present as biochemical relapses, defined as an increase in serum prostate specific antigen (PSA) level (4). In patients with biochemical recurrence, functional imaging with 18F-fluorocholine (F-choline) or, more recently, 68Ga-prostate-specific membrane antigen (PSMA) PET/CT, plays a key role in distinguishing a local recurrence from a systemic spread of the disease, in order to optimize the therapeutic strategy (5,6). In fact, it has been shown that the results of PET/CT could influence patient’s management in more than half cases (7-9).
F-choline is a choline analogue, mimicking choline uptake and phosphorylation as a precursor in the biosynthesis of phosphatidylcholine, a membrane phospholipid (10). Physiological uptake of F-choline is noted in kidneys, liver, salivary glands, pancreas and with a weaker intensity in spleen, bone marrow and muscles. Increased uptake has been documented in benign lesions such as adrenal and parathyroid adenomas, meningiomas, sarcoidosis lesions or thymomas (10-13). Finally, increased choline kinase activity has been demonstrated in a wide variety of human malignancies (10,14) such as PCa (5,15), hepatocellular carcinoma (16) or bronchioloalveolar lung cancer (17). Currently only a few large series investigated the potential of F-choline PET/CT to detect second malignancies in patients referred for the staging of a PCa (14,18).
The aim of our study was therefore to describe and analyze unusual F-choline uptakes in a large series of PCa patients who underwent F-choline PET/CT for the initial staging of their disease, or for a restaging after a biochemical relapse.
Methods
Population
We retrospectively identified all adult patients (≥18 years old) who underwent F-choline PET/CT between January 2012 and March 2019 within the Nuclear Medicine Department at Besançon University Hospital. Among these patients, we included those referred for the initial staging of a PCa (because of suspicious findings on conventional imaging) or for the evaluation of a biological recurrence of a histologically proven PCa initially treated by surgery and/or radiotherapy with or without androgen deprivation therapy. Among included patients, we identified those presenting with an unusual tracer uptake, defined as a F-choline uptake outside the expected areas of PCa dissemination, i.e., the prostate and seminal vesicles, pelvic nodes, retroperitoneal nodes up to L2–L3, and bones (19-21), or as F-choline uptake in bone with a clear morphological evidence of nonmetastatic lesion.
F-choline PET/CT acquisition technique
All patients fasted for at least 4 hours before receiving an intravenous injection of 4 MBq/kg of F-choline. Slow hydration with normal saline was delivered to all patients between injection and acquisition. Acquisitions were performed on a GE DISCOVERY 690 PET/CT (GE Healthcare, Milwaukee, WI, USA). The acquisition protocol included a dynamic acquisition centered over the pelvis for 8 min with 1-min frames, followed by an acquisition from vertex to mid-thigh performed 45 min after injection, comprising 7–8 bed positions with an acquisition time per bed position varying between 1.5 and 2.5 min, depending on the patient’s body mass index. PET images were reconstructed using a standard iterative algorithm. A “low-dose” CT (50 to 210 mA, 120 kV, 3.75 mm slice thickness) was performed for attenuation correction of the PET data for both dynamic and late acquisitions (22,23). Images were interpreted by two nuclear medicine specialists on a dedicated Advantage Workstation console (GE Healthcare, Milwaukee, WI, USA). All the relevant clinical, histological and radiological data were collected from the electronic hospital charts of the patients, analyzed and reported in this study.
This study obtained ethics approval from our Institutional Review Board, with waiver of informed consent for this retrospective study.
Results
Population
Three hundred and sixty-eight patients were included in this study. Table 1 summarizes the main characteristics of our population. Unusual uptakes were found in 47/368 patients (12.8%). Table 2 summarizes these unusual uptakes, as well as their associated maximum standardized uptake values (SUVmax).

Full table
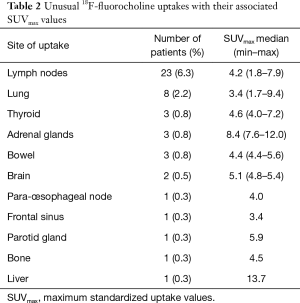
Full table
Nodal uptake
We found unusual nodal uptake in 23 patients (6.3%). Median age was 71 years (range, 63–81 years), and the median PSA was 3 ng/mL (range, 0.2–14 ng/mL). Twenty-two cases were finally considered as benign inflammatory mediastino-hilar (n=12), inguinal (n=5), axillary (n=4) and cervical (n=1) lymph nodes. Histological evidence was available for two patients who underwent a biopsy. For the other 20 patients, diagnosis was made on the basis of clinical, imaging, biological and follow up data. Only one patient had a single nodal uptake. According to the D’Amico classification (2), eight patients presented with a low-risk PCa at initial diagnosis, 9 had an intermediate-risk cancer (6 with a 3+4 Gleason score and 3 with a 4+3 Gleason score) and 5 had a high-risk cancer.
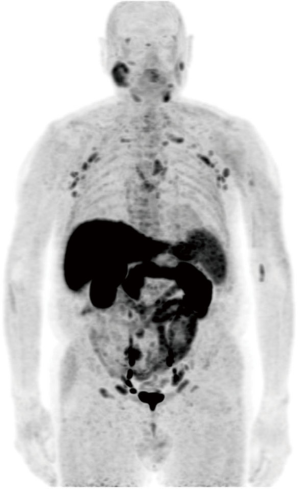
The last case corresponded to an 81-year-old patient who underwent prostatectomy in 2012 for a localized PCa (initial PSA: 3.3 ng/mL, Gleason score 3+3, pT2cN0M0), and relapsed in 2018 (PSA level: 0.21 ng/mL). F-choline PET/CT demonstrated a local recurrence in the prostate bed as well as multiple hypermetabolic lymph nodes above and below the diaphragm, corresponding to a marginal zone B-cell lymphoma (Figure 1).
Lung uptake
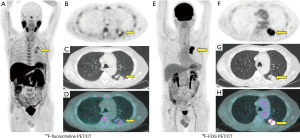
We found unusual lung uptake in 8 patients (2.2%). Median age was 67 years (range, 58–80 years), and the median PSA was 1.7 ng/mL (range, 0.9–7.5 ng/mL). Four cases corresponded to non-small cell lung cancers (3 adenocarcinomas and 1 squamous cell carcinoma). Figure 2 provides an illustrating example. For two patients, lung cancer was already diagnosed at the time of the PET/CT procedure, so these patients cannot be considered as true incidental findings. For the other two patients, lung cancer was revealed by 18F-fluorocholine PET/CT.
Three patients presented with hypermetabolic isolated pulmonary nodules (median SUVmax =1.7, range, 1.1–4.1) that did not evolve morphologically on subsequent CT scans in the absence of systemic hormonal treatment. For this reason, they were classified as benign lesions.
The last patient in this subgroup was an 80-year-old man initially treated with prostatectomy in 2002 for a localized intermediate risk PCa (initial PSA: 4.3 ng/mL, Gleason score 4+3 =7, pT2bN0M0) who underwent PET/CT because of a rising PSA up to 3.4 ng/mL. F-choline PET/CT revealed diffuse hypermetabolic ground-glass opacities of the lung (SUVmax =2.8) with bronchial distortions and intra-lobular crosslinking without further abnormalities. This aspect was attributed to an extrinsic allergic alveolitis known as “farmer’s lung” already recognized in the medical history of the patient. This disease is due to chronic inhalation of microorganisms living in hay, straw or moldy cereals (24).
Thyroid uptake
We found unusual thyroid uptake in 3 patients (0.8%). Median age was 69 years (range, 61–71 years), and the median PSA was 1.3 ng/mL (range, 0.2–1.7 ng/mL). Two of them displayed a diffuse uptake suggestive of thyroiditis, a diagnosis confirmed by subsequent morphological and biological data. The third patient presented with a focal uptake corresponding to a hypodense nodule in the right thyroid lobe (SUVmax =7.2). Ultrasonography demonstrated a well-defined, hypoechoic, hyper-vascular nodular lesion of the right thyroid lobe (diameter: 30 mm). Three needle biopsies under ultrasound guidance were performed, and no signs of malignancy were found. This patient is still alive without evidence of thyroid carcinoma and presents a normal thyroid function.
Adrenal uptake
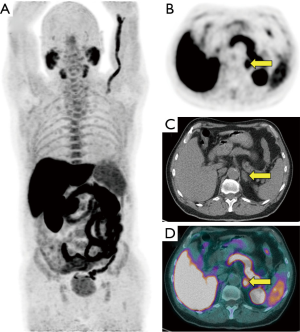
We found unusual unilateral adrenal uptake in 3 patients (0.8%) who underwent F-choline PET/CT for biological relapse after prostatectomy (n=1) or radiotherapy (n=2). Median age was 79 years (range, 66–80 years), and the median PSA was 1.3 ng/mL (range, 0.5–2.2 ng/mL). Two of them showed highly hypermetabolic supra-centimetric nodular lesions in the left adrenal gland (SUVmax =7.6 and 12). CT-guided biopsies revealed healthy adrenal tissue without atypia in both cases. These patients are still alive with stable PCa disease 52 and 61 months after F-choline PET/CT. Figure 3 illustrates one of these cases. The third patient also presented with a focal uptake in a nodular lesion (SUVmax =8.4) of the left adrenal gland but no biopsy was done given the patient’s age. A CT scan performed 25 months after the PET/CT did not reveal any morphological evolution of this lesion, in the absence of androgen deprivation therapy.
Colic uptake
We found unusual colic uptake in 3 patients (0.8%). Median age was 79 years (range, 66–80 years), and the median PSA was 1.76 ng/mL (range, 0.5–2.2 ng/mL). Two of them presented with hypermetabolic foci in the recto-sigmoid, while the third patient displayed a more diffuse uptake of the left colon. Median SUVmax was 4.9 (range, 4.4–5.6). There was no evident morphological abnormality of the intestinal wall on CT images. Unfortunately, none of these patients underwent an endoscopy. However, they did not develop any digestive neoplasia after 3 years of follow-up for two patients (one with focal uptake, one with diffuse uptake), and after 18 months of follow-up for one patient (with focal uptake).
Cerebral uptake
In two patients, we observed an intense focal uptake within the cerebral parenchyma. First case corresponded to a meningioma (Figure 4). The patient has not been operated on and has been followed with annual brain magnetic resonance imaging (MRI) demonstrating a slow growth of the meningioma. The second patient displayed a focal meningeal uptake close to the sella turcica and unfortunately declined brain MRI because of claustrophobia. He does not complain from neurological symptoms 5 years later.

Para-esophageal uptake
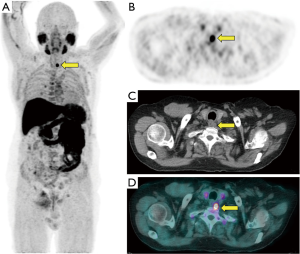
We found an unusual para-esophageal nodal uptake in a 68-year-old patient initially treated in 2010 with radiotherapy and 3 years of androgen deprivation therapy for a high risk PCa (initial PSA: 19 ng/mL, Gleason score: 4+5 =9, pT2cN0M0) and presenting with a biochemical relapse (PSA: 0.7 ng/mL). F-choline PET/CT demonstrated a para-esophageal hypermetabolic adenopathy (SUVmax =4), without evidence of PCa recurrence elsewhere (Figure 5). Biopsy of this lesion established the diagnosis of metastatic adenopathy of a well-differentiated grade 1 neuroendocrine tumor. Whole body CT scan and 111In-octreotide scan confirmed an isolated hyper-arterialized hyper-fixing adenopathy. Biochemistry showed a normal neuron-specific enolase (NSE) blood concentration and high levels of chromogranin A (17,277 ng/mL). This lesion was treated with exclusive radiotherapy (60 Grays in 30 fractions), as surgery was excluded because of important patient co-morbidities. The patient underwent an 111In-octreotide scan in 2018 and a F-choline PET/CT in 2019, with no sign of PCa or neuroendocrine tumor relapse. The patient is still alive 45 months after the diagnosis of neuroendocrine tumor.
Frontal sinus uptake
An 82-year-old patient, treated with radiotherapy and androgen deprivation therapy in 2016 for a localized PCa (initial PSA: 13 ng/mL, Gleason score 3+4 =7, pT2cN0M0), underwent F-choline PET/CT in 2018 for a biochemical relapse (PSA: 1.9 ng/mL). This patient did not show any sign of local or systemic PCa relapse, but presented with an unusual uptake (SUVmax =3.4) in the left frontal sinus, without bone erosion. This anomaly was attributed to a banal infectious sinusitis, as it disappeared on subsequent CT scans obtained during follow-up of the patient.
Parotid uptake
A 79-year-old patient, treated with prostatectomy in 2012 for a localized intermediate risk PCa (initial PSA: 8 ng/mL, Gleason score 4+3 =7, pT2bN0M0), underwent F-choline PET/CT in 2018 for a biochemical relapse (PSA =27 ng/mL). In this patient we found a retroperitoneal nodal relapse of the PCa and a clear asymmetry of fixation between the right (SUVmax =6) and left parotid gland (SUVmax =3.7). CT scan showed a significant increase in the right parotid volume without obvious morphological lesion within the gland. There is no definite diagnosis for this anomaly. Follow-up CT scans showed a normalization of the right parotid gland size without hormone therapy. The patient was still alive 17 months later.
Bone uptake
A 79-year-old patient, treated with radiotherapy and androgen deprivation therapy in 2007 for a localized intermediate risk PCa (initial PSA: 6.8 ng/mL, Gleason score: 4+3 =7, pT2aN0M0) underwent F-choline PET/CT in 2016 for a biochemical relapse (PSA: 2.58 ng/mL). This examination showed a diffuse moderate uptake in the left hemi-pelvis (SUVmax =2.7) and in the 2nd lumbar vertebral body (SUVmax =4.2), with a typical trabecular aspect of the bone on CT images. These lesions were related to a Paget’s disease, already known in the patient’s history. There was no sign of PCa relapse. Because of a persistent PSA elevation, this patient underwent a 68Ga-PSMA PET/CT in 2017, which demonstrated a nodal pelvic and lombo-aortic relapse, without any sign of bone metastases.
Liver uptake
A 58-year-old patient, treated with radical prostatectomy in 2018 for a localized PCa (initial PSA: 6.4 ng/mL, Gleason score: 4+4 =8, pT2cN0M0), underwent F-choline PET/CT in 2018 for a persistent high PSA level after surgery (PSA: 2.1 ng/mL). This examination showed a focal uptake in a peri-rectal nodular lesion suspected to correspond to a seminal vesicle remnant. There was also a focal uptake (SUVmax =13.7) in a hypodense lesion of the liver. This anomaly was explored with MRI and contrast-enhanced ultrasonography, both in favor of a hepatic angioma, but the ultrasonography data were ambiguous and the patient eventually underwent biopsy: the results were in favor of a cavernous hemangioma, with absence of any malignant structure.
Discussion
In our population of 368 patients who underwent F-choline PET/CT, we found atypical, non-PCa related uptake in 47 patients (12.8%). Our results are comparable to those published by Calabria et al. (14), who described abnormal F-choline uptake in a population of 300 patients referred for a relapsing PCa and found 48 cases (16.0%) of tracer uptake not related to PCa.
Lymph nodes are, in our study, the most common site of unusual tracer accumulation, accounting for almost 50% of cases in our population. They largely predominated in the mediastinal, inguinal and axillary areas. Our results support data available in the literature (14,15,18). Most cases of nodal uptake have been considered to be of inflammatory origin, and increased F-choline uptake during inflammation could be related to the activation of macrophages (25). In fact, it has been reported that in inflammatory conditions extracellular signals can activate the transcription of the gene coding for CTL1 (choline transporter-like protein 1), a membrane transporter of choline, in macrophages. Increased CTL1 expression is associated to an increase in phosphatidylcholine synthesis (25). Phosphatidylcholine is involved in many biochemical pathways in macrophages, including the production of pro-inflammatory cytokines such as TNF-α or IL-6 (25,26). These data could also explain the likely inflammatory uptake that we observed in the frontal sinus of one patient. Finally, alveolar macrophages activation plays a key role in the development of “farmer’s lung” allergic alveolitis, promoting the recruitment of T lymphocytes in the lung parenchyma (27).
F-choline PET/CT revealed diffuse hypermetabolic adenopathies in a patient suffering from an indolent marginal zone B-cell lymphoma. F-choline uptake in different lymphoma subtypes has already been reported (28,29), in connection with tumor cell overexpression of choline kinase alpha (30). Overexpression of choline kinase and phosphorylcholine-cytidyl transferase has also been documented in different subtypes of lung cancer (18,31). In our population, two cases of lung cancer were unknown before F-choline PET/CT and this examination clearly influenced the therapeutic approach for these patients. Based on these results, we believe that it is necessary to establish a definite diagnosis for any hypermetabolic pulmonary lesion on F-choline PET/CT.
We found 3 patients presenting with diffuse or focal F-choline uptake in the thyroid, and all were finally considered as benign diseases. These results are similar to those of Calabria et al. (11) who found 2 out of 300 patients with diffuse uptake due to thyroiditis. However, a retrospective study of 368 patients focusing exclusively on abnormal F-choline uptake in the thyroid gland at PET/CT found two cases of thyroid carcinoma out of nine cases of atypical thyroid uptake (32). Case reports also demonstrated that focal uptake of F-choline in the thyroid should raise suspicion for malignancy (33-35). Thus, in agreement with the conclusion of a recent literature review evaluating the prevalence and clinical significance of focal incidental radiolabeled choline uptake in the thyroid gland (36), any abnormal thyroid uptake at F-choline PET/CT should be explored with at least a TSH and calcitonin assay and an ultrasonography.
Three patients presented with atypical adrenal uptake in our cohort, none of them related to a malignant tumor. These data are consistent with the literature (14,15,18). Adrenal uptake may be stimulated by acute stress such as cold exposure prior to examination, which has been shown to increase choline acetyltransferase activity in a rat model (37). Adrenal adenomas can appear hypermetabolic on F-choline PET/CT (38). Of note, one case report has been published concerning an adrenal metastasis of PCa diagnosed at F-choline PET/CT (39).
Overexpression of choline kinase has been documented in colon cancers (40,41) as well as in colic adenomas (14). As a consequence, a colonoscopy should have been performed in patients demonstrating atypical colic uptake. Unfortunately, none of our patients underwent an endoscopy, but none of them developed a colorectal cancer during follow-up.
Asymmetrical F-choline uptake in salivary glands can be due to asymmetric development of the glands, as frequently observed for the submandibular glands. It may also be due to intraglandular lithiasis which can cause a lack of tracer uptake in the affected gland (42). However, in our case the affected parotid demonstrated a transient enlargement with hypermetabolism, and we speculate that it could have resulted from an acute episode of sialadenitis (43).
Concerning unusual brain uptakes, F-choline uptake has already been reported in meningiomas as well as in glioblastomas or brain metastases (14,44,45). Of note, F-choline uptake was faint in grade II and III gliomas (44). Pathophysiology of increased choline uptake in these tumors is poorly documented and may involve an increase in cell membrane synthesis.
Finally, F-choline uptake in well-differentiated neuroendocrine tumors has been described in various contexts including isolated pulmonary nodule (46) and metastatic mediastinal and cervical lymph nodes from gastro-entero-pancreatic neuroendocrine tumor (47).
Some limitations should be acknowledged in our study. First, this is a retrospective study and not all atypical uptakes can be considered as true incidentalomas. Then, we do not have a pathological proof in most cases, but a definite diagnosis could be established for 17 patients based on biological, imaging and/or pathological data (there is no need for a biopsy when diagnosing a thyroiditis, for example). Among these 17 patients we found a malignant lesion in 6 patients. For the remaining 30 patients, a diagnosis of benign anomaly was reasonably assumed based on clinical and/or imaging follow-up.
Conclusions
Atypical F-choline uptake on PET/CT occurred in 12.8% of our patients (47/368), with only 6 cases of malignant lesion. Despite the fact that most unusual F-choline uptakes are benign, they should be explored in order to not miss a non-PCa.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/qims-19-981). Dr. AC serves as an unpaid editorial board member of Quantitative Imaging in Medicine and Surgery. The other authors have no conflicts of interest to declare.
Ethical Statement: The study was conducted in accordance with the Declaration of Helsinki. This study obtained ethics approval from our Institutional Review Board, with waiver of informed consent for this retrospective study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- D’Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, Tomaszewski JE, Renshaw AA, Kaplan I, Beard CJ, Wein A. Biochemical Outcome After Radical Prostatectomy, External Beam Radiation Therapy, or Interstitial Radiation Therapy for Clinically Localized Prostate Cancer. JAMA 1998;280:969-74. [Crossref] [PubMed]
- Liu J, Zhao J, Zhang M, Chen N, Sun G, Yang Y, Zhang X, Chen J, Shen P, Shi M, Zeng H. The validation of the 2014 International Society of Urological Pathology (ISUP) grading system for patients with high-risk prostate cancer: a single-center retrospective study. Cancer Manag Res 2019;11:6521-9. [Crossref] [PubMed]
- Dong JT, Rinker-Schaeffer CW, Ichikawa T, Barrett JC, Isaacs JT. Prostate cancer--biology of metastasis and its clinical implications. World J Urol 1996;14:182-9. [Crossref] [PubMed]
- De Bari B, Alongi F, Lestrade L, Giammarile F. Choline-PET in prostate cancer management: The point of view of the radiation oncologist. Crit Rev Oncol Hematol 2014;91:234-47. [Crossref] [PubMed]
- Treglia G, Annunziata S, Pizzuto DA, Giovanella L, Prior JO, Ceriani L. Detection Rate of 18F-Labeled PSMA PET/CT in Biochemical Recurrent Prostate Cancer: A Systematic Review and a Meta-Analysis. Cancers 2019;11:710. [Crossref] [PubMed]
- Cornford P, Bellmunt J, Bolla M, Briers E, De Santis M, Gross T, Henry AM, Joniau S, Lam TB, Mason MD, Van der Poel HG, Van der Kwast TH, Rouvière O, Wiegel T, Mottet N. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: Treatment of Relapsing, Metastatic, and Castration-Resistant Prostate Cancer. Eur Urol 2017;71:630-42. [Crossref] [PubMed]
- Kirienko M, Sollini M, Lopci E, Versari A, Chiti A. Applications of PET imaging with radiolabelled choline (11C/18F-choline). Q J Nucl Med Mol Imaging 2015;59:83-94. [PubMed]
- Pasqualetti F, Panichi M, Sainato A, Matteucci F, Galli L, Cocuzza P, Ferrazza P, Coraggio G, Pasqualetti G, Derosa L, Sollini M, Mannelli L, Ortori S, Monzani F, Ricci S, Greco C, Fabrini MG, Erba PA. [18F]Choline PET/CT and stereotactic body radiotherapy on treatment decision making of oligometastatic prostate cancer patients: preliminary results. Radiat Oncol 2016;11:9. [Crossref] [PubMed]
- Vallabhajosula S. 18F-Labeled Positron Emission Tomographic Radiopharmaceuticals in Oncology: An Overview of Radiochemistry and Mechanisms of Tumor Localization. Semin Nucl Med 2007;37:400-19. [Crossref] [PubMed]
- Calabria F, D'Auria S, Sannino P, Schillaci O. A case of thymoma detected by 18F-choline positron emission tomography/computed tomography. Eur J Nucl Med Mol Imaging 2011;38:602. [Crossref] [PubMed]
- Takesh M, Haberkorn U, Strauss LG, Roumia S, Dimitrakopoulou-Strauss A. Incidental detection and monitoring of spontaneous recovery of sarcoidosis via fluorine-18-fluoroethyl-choline positron emission tomography/computed tomography. Hell J Nucl Med 2012;15:63-5. [PubMed]
- Mapelli P, Busnardo E, Magnani P, Freschi M, Picchio M, Gianolli L, Messa C. Incidental finding of parathyroid adenoma with 11C-choline PET/CT. Clin Nucl Med 2012;37:593-5. [Crossref] [PubMed]
- Calabria F, Chiaravalloti A, Schillaci O. 18F-Choline PET/CT Pitfalls in Image Interpretation: An Update on 300 Examined Patients with Prostate Cancer. Clin Nucl Med 2014;39:122. [Crossref] [PubMed]
- Schillaci O, Calabria F, Tavolozza M, Cicciò C, Carlani M, Caracciolo CR, Danieli R, Orlacchio A, Simonetti G. 18F-choline PET/CT physiological distribution and pitfalls in image interpretation: experience in 80 patients with prostate cancer. Nucl Med Commun 2010;31:39. [Crossref] [PubMed]
- Talbot JN, Fartoux L, Balogova S, Nataf V, Kerrou K, Gutman F, Huchet V, Ancel D, Grange JD, Rosmorduc O. Detection of hepatocellular carcinoma with PET/CT: a prospective comparison of 18F-fluorocholine and 18F-FDG in patients with cirrhosis or chronic liver disease. J Nucl Med 2010;51:1699-706. [Crossref] [PubMed]
- Balogova S, Huchet V, Kerrou K, Nataf V, Gutman F, Antoine M, Ruppert AM, Prignon A, Lavolée A, Montravers F, Mayaud C, Cadranel J, Talbot JN. Detection of bronchioloalveolar cancer by means of PET/CT and 18F-fluorocholine, and comparison with 18F-fluorodeoxyglucose. Nucl Med Commun 2010;31:389. [Crossref] [PubMed]
- Sollini M, Pasqualetti F, Perri M, Coraggio G, Castelluci P, Roncali M, Boni R, Lazzeri E, Galeandro M, Paiar F, Versari A, Erba PA. Detection of a second malignancy in prostate cancer patients by using [18F]Choline PET/CT: a case series. Cancer Imaging 2016;16:27. [Crossref] [PubMed]
- Parker WP, Davis BJ, Park SS, Olivier KR, Choo R, Nathan MA, Lowe VJ, Welch TJ, Evans JD, Harmsen WS, Zaid HB, Sobol I, Moreira DM, Haloi R, Tollefson MK, Gettman MT, Boorjian SA, Mynderse LA, Karnes RJ, Kwon ED. Identification of Site-specific Recurrence Following Primary Radiation Therapy for Prostate Cancer Using C-11 Choline Positron Emission Tomography/Computed Tomography: A Nomogram for Predicting Extrapelvic Disease. Eur Urol 2017;71:340-8. [Crossref] [PubMed]
- Lépinoy A, Cochet A, Cueff A, Cormier L, Martin E, Maingon P, Bosset JF, Brunotte F, Créhange G. Pattern of occult nodal relapse diagnosed with 18F-fluoro-choline PET/CT in prostate cancer patients with biochemical failure after prostate-only radiotherapy. Radiother Oncol 2014;111:120-5. [Crossref] [PubMed]
- Hövels AM, Heesakkers RAM, Adang EM, Jager GJ, Strum S, Hoogeveen YL, Severens JL, Barentsz JO. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin Radiol 2008;63:387-95. [Crossref] [PubMed]
- Heinisch M, Dirisamer A, Loidl W, Stoiber F, Gruy B, Haim S, Langsteger W. Positron Emission Tomography/Computed Tomography with F-18-fluorocholine for Restaging of Prostate Cancer Patients: Meaningful at PSA < 5 ng/ml? Mol Imaging Biol 2006;8:43-8. [Crossref] [PubMed]
- Bakhsh A, Venel Y, Courtehoux M, Maia S, Perault C, Santiago-Ribeiro MJ, Erra B. 18-Fluorocholine PET/CT in prostate cancer: Which acquisition protocol? Médecine Nucléaire 2016;40:307-14. [Crossref]
- Cano-Jiménez E, Rubal D, Pérez de Llano LA, Mengual N, Castro-Añón O, Méndez L, Golpe R, Sanjuán P, Martín I, Veres A. Farmer's lung disease: Analysis of 75 cases. Med Clin (Barc) 2017;149:429-35. [PubMed]
- Snider SA, Margison KD, Ghorbani P, LeBlond ND, O'Dwyer C, Nunes JRC, Nguyen T, Xu H, Bennett SAL, Fullerton MD. Choline transport links macrophage phospholipid metabolism and inflammation. J Biol Chem 2018;293:11600-11. [Crossref] [PubMed]
- Sanchez-Lopez E, Zhong Z, Stubelius A, Sweeney SR, Booshehri LM, Antonucci L, Liu-Bryan R, Lodi A, Terkeltaub R, Lacal JC, Murphy AN, Hoffman HM, Tiziani S, Guma M, Karin M. Choline Uptake and Metabolism Modulate Macrophage IL-1β and IL-18 Production. Cell Metab 2019;29:1350-1362.e7. [Crossref] [PubMed]
- Lacasse Y, Israël Assayag E, Laviolette M, Cormier Y. Clinical and immunopathological aspects of hypersensitivity pneumonitis. Revue des Maladies Respiratoires 2004;21:769-81. [Crossref] [PubMed]
- Garzon JG, Bassa P, Moragas M, Soler M, Riera E. Incidental diagnosis of diffuse large B-cell lymphoma by 11C-choline PET/CT in a patient with biochemical recurrence of prostate cancer. Clin Nucl Med 2014;39:742-3. [Crossref] [PubMed]
- Goineau A, Supiot S. Incidental Detection of a Hodgkin Lymphoma on 18F-Choline PET/CT and Comparison With 18FDG PET/CT in a Patient with Prostate Cancer Clin Nucl Med 2016;41:746. Reply. [Crossref] [PubMed]
- Xiong J, Bian J, Wang L, Zhou JY, Wang Y, Zhao Y, Wu LL, Hu JJ, Li B, Chen SJ, Yan C, Zhao WL. Dysregulated choline metabolism in T-cell lymphoma: role of choline kinase-α and therapeutic targeting. Blood Cancer J 2015;5:287. [Crossref] [PubMed]
- Li M, Peng Z, Liu Q, Sun J, Yao S, Liu Q. Value of 11C-choline PET/CT for lung cancer diagnosis and the relation between choline metabolism and proliferation of cancer cells. Oncol Rep 2013;29:205-11. [Crossref] [PubMed]
- Albano D, Durmo R, Bertagna F, Giubbini R. 18F-choline PET/CT incidental thyroid uptake in patients studied for prostate cancer. Endocrine 2019;63:531-6. [Crossref] [PubMed]
- Lalire P, Zalzali M, Garbar C, Bruna-Muraille C, Morland D. Incidental detection of oxyphilic papillary thyroid carcinoma by 18F-fluorocholine PET/CT. Clin Nucl Med 2016;41:512-3. [Crossref] [PubMed]
- Ouattara A, Ribeiro de Oliveira T, Holz S, Van den Bossche H, Strybol D, Assenmacher C, Everaerts W, De Meerleer G, Joniau S. Incidental detection of occult thyroid carcinoma with 11C-Choline PET/CT for high risk prostate cancer. Curr Urol 2017;10:217-20. [Crossref] [PubMed]
- Ciappuccini R, Edet-Sanson A, Saguet-Rysanek V, Gauthé M, Bardet S. Thyroid incidentaloma on 18F-fluorocholine PET/CT and 68Ga-PSMA PET/CT revealing a medullary thyroid carcinoma. Clin Nucl Med 2019;44:663-5. [Crossref] [PubMed]
- Bertagna F, Albano D, Giovanella L, Giubbini R, Treglia G. F18-choline/C11-choline PET/CT thyroid incidentalomas. Endocrine 2019;64:203-8. [Crossref] [PubMed]
- Wahba ZZ, Soliman KFA. Effect of stress on choline acetyltransferase activity of the brain and the adrenal of the rat. Experientia 1992;48:265-8. [Crossref] [PubMed]
- Imperiale A, Facundo Cabral J, Rust E, Flores-Turk G, Renard C, Hubele F, Detour J, Lang H, Gangi A, Namer IJ. 18F-fluorocholine uptake in a case of adrenal incidentaloma: possible diagnostic pitfall or potential tool for adrenocortical tumors characterization? Clin Nucl Med 2013;38:e83-4. [Crossref] [PubMed]
- Matrone F, Sivolella S, Bellavita R, Casciola L, Cristallini EG, Aristei C. Use of 18F-choline positron emission tomography/CT in high-risk prostate cancer: a case of solitary adrenal metastasis. Tumori 2015;101:e21-3. [Crossref] [PubMed]
- Ramírez de Molina A, Rodríguez-González A, Gutiérrez R, Martínez-Piñeiro L, Sánchez J, Bonilla F, Rosell R, Lacal J. Overexpression of choline kinase is a frequent feature in human tumor-derived cell lines and in lung, prostate, and colorectal human cancers. Biochem Biophys Res Commun 2002;296:580-3. [Crossref] [PubMed]
- Nakagami K, Uchida T, Ohwada S, Koibuchi Y, Suda Y, Sekine T, Morishita Y. Increased Choline Kinase Activity and Elevated Phosphocholine Levels in Human Colon Cancer. Jpn J Cancer Res 1999;90:419-24. [Crossref] [PubMed]
- Clarençon F, Kerrou K, Gutman F, Chevallier D, Montravers F, Talbot JN. Asymmetric F-18 fluorocholine uptake of submaxillary glands revealing intraglandular lithiasis. Clin Nucl Med 2007;32:165-7. [Crossref] [PubMed]
- Wilson KF, Meier JD, Ward PD. Salivary gland disorders. Am Fam Physician 2014;89:882-8. [PubMed]
- Mertens K, Ham H, Deblaere K, Okito Kalala JP, Van den Broecke C, Slaets D, De Vos F, Goethals I. Distribution Patterns of 18F-Labelled Fluoromethylcholine in Normal Structures and Tumors of the Head: A PET/MRI Evaluation. Clin Nucl Med 2012;37:e196. [Crossref] [PubMed]
- Fallanca F, Giovacchini G, Picchio M, Bettinardi V, Messa C, Fazio F. Incidental detection by [11C]choline PET/CT of meningiomas in prostate cancer patients. Q J Nucl Med Mol Imaging 2009;53:417-21. [PubMed]
- Treglia G, Lococo F, Petrone G, Inzani F, Perotti G, Porziella V, Granone P, Rindi G, Giordano A, Rufini V. Pulmonary neuroendocrine tumor incidentally detected by 18F-CH PET/CT. Clin Nucl Med 2013;38:e196-9. [Crossref] [PubMed]
- Kavanal AJ, Bhadada SK, Sood A, Kaur G, Parwaiz A, Gulati A, Dahiya D, Mittal BR. Triple Tracer Positivity in Metastatic Lymph Nodes from Well-Differentiated Neuroendocrine Tumor in MEN1 Syndrome. J Nucl Med Technol 2020. Epub ahead of print. [Crossref] [PubMed]


