The role of magnetic resonance imaging in hypertrophic cardiomyopathy
Introduction
Hypertrophic cardiomyopathy (HCM) is the most common inheritable cardiac disorder, with an estimated prevalence of 1:500 in the general population (1,2). The mode of inheritance is autosomal dominant in approximately 50-60% of cases with over 600 mutations identified in sarcomeric genes to date (2). These mutations are thought to cause an increase in myocyte stress and impaired function that eventually leads to left ventricular hypertrophy (LVH) and fibrosis. The key histologic feature of HCM is myocyte and myofibrillar disarray with a non-linear or haphazard arrangement of myocytes by light microscopy (3). There is a wide range of penetrance and phenotypic expression due to the genetic diversity together with modifier genes and environmental factors with asymmetric involvement of the interventricular septum being the most common pattern. Most cases of HCM are phenotypically expressed in adolescence or early adulthood but age-related penetrance with certain phenotypes is increasingly recognized whereby there can be delayed emergence of LVH in midlife and beyond (4). Clinical manifestations of HCM are wide ranging but are usually the result of systolic and/or diastolic dysfunction, left ventricular outflow tract (LVOT) obstruction, supraventricular/ventricular arrhythmias and sudden cardiac death (SCD) (1). In recent years magnetic resonance imaging (MRI) has become established as an important tool for the evaluation of suspected HCM as it can reliably establish the diagnosis, help distinguish HCM from other causes of LVH and identify those patients at greatest risk of SCD. This article reviews the current status of MRI in the evaluation of the HCM patient including imaging protocols, disease characterization and the emerging role of MRI for risk stratification and proband screening.
Non-invasive imaging techniques
Traditionally, the diagnosis of HCM relies upon clinical assessment and transthoracic echocardiography (TTE) to identify unexplained LVH in the presence of a non-dilated LV cavity (5). Other important imaging features which are supportive of a diagnosis of HCM are systolic anterior motion (SAM) of the mitral valve, and LVOT obstruction. In many cases TTE assessment is unable to confidently establish or refute a diagnosis of HCM due to a combination of technical limitations and the highly variable nature of HCM phenotypic expression (6). In recent years MRI has become established as a useful adjunct to TTE owing to its unrestricted field of view, more accurate measurement of LV wall thickness, mass, volumes and function and its ability to provide non-invasive assessment of myocardial fibrosis. In 10-15% of HCM patients, there is focal segmental LV hypertrophy typically limited to the anterolateral free wall, posterior septum, or apex, which are technically challenging areas for TTE, due to limitations of imaging windows (7). A recent study by Moon et al. found that ten patients with negative findings for apical HCM on TTE had positive MRI findings for apical HCM, including one patient having an LV wall thickness of 28 mm on MRI (6). Due to the growing evidence-based practice most cardiac imaging centres now routinely perform MRI in all new patients with suspected HCM as endorsed by the American Society of Echocardiography 2011 consensus guidelines (5).
Cardiac MRI technique
At our centre cardiac MRI is performed using a 1.5 Tesla clinical system (Ingenia, Philips Healthcare, Best, The Netherlands). We use a standard HCM protocol with addition of flow sensitive sequences and stress perfusion imaging in selected cases (Table 1) (8). Cine imaging with bright blood prepared steady state free precession (SSFP) sequences provides intrinsically high definition of the blood pool-myocardium interface and forms the basis of morphological assessment. A complete set of SSFP sequences acquired in the short axis plane from base to apex enable identification and measurement of hypertrophied regions, LV volumes, ejection fraction and LV mass (using semi-automated post processing software). SSFP images in standardised 2-, 3- and 4-chamber planes provide additional morphological assessment. Late gadolinium enhancement (LGE) imaging provides non-invasive tissue characterization by identification of HCM associated interstitial and replacement fibrosis. For LGE imaging the patient is administered an intravenous injection of 0.2 mmol per kilogram of body weight gadolinium based contrast agent (Gadovist 1.0, Bayer Schering Pharma, Berlin-Wedding, Germany) at 1-2 mL/s. Ten minutes after injection time LGE images are acquired in the continuous short-axis view using an inversion-recovery gradient-recalled echo sequence using a manually selected optimal inversion time to null the signal from normal myocardium (9).
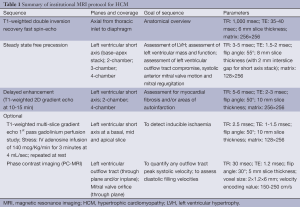
Full table
LGE in HCM
LGE is recognised with many disease processes that involve the myocardium including myocardial infarction, myocarditis and various cardiomyopathic processes (10). Common to these conditions is the accumulation and delayed wash-out of gadolinium chelates from regions of expanded extracellular space (10-12). The precise pathophysiologic mechanism responsible for LGE in HCM remains uncertain but observations from imaging and histologic studies suggest that LGE may derive from a pathophysiologic cascade in which repetitive bouts of microvascular ischemia result from structurally abnormal intramural coronary arteries leading to myocyte cell death and repair in the form of replacement fibrosis. An alternative hypothesis suggests the causative sarcomeric gene mutations may directly cause increased myocardial connective tissue deposition. Moon et al. showed a direct correlation between the percentage of LGE and percentage of histologic collagen in an explanted HCM heart (12). LGE has been reported in up to 75% of patients with HCM in whom the vast majority have patchy mid-wall-type enhancement which is typically most pronounced within the segments most severely affected by hypertrophy (13). LGE most often involves the interventricular septum, particularly the anteroseptal mid to basal segments and right ventricular insertion points (14).
Disease characterization
HCM phenotypes
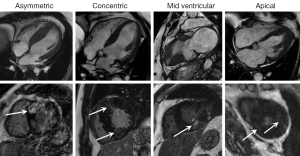
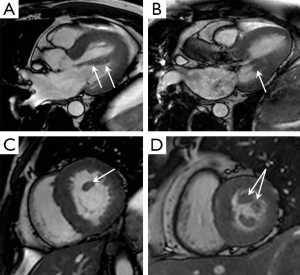
Phenotypic heterogeneity causes great variability in the imaging appearances of HCM which can present significant diagnostic challenges when trying to establish the diagnosis (15). The commonest HCM phenotype is asymmetric septal hypertrophy (approximately 70% of cases) with a septal to posterior wall ratio of >1:3 considered diagnostic (5). So called “atypical” phenotypes include apical, mid-ventricular, concentric and focal forms (15). MRI has particular utility in these variants owing to its complete unrestricted coverage of the LV, especially when disease is confined to just a few myocardial segments separated by regions of normal wall thickness, when TTE may struggle to provide definitive characterization (Figure 1). It is important to note that LV mass will often be within the normal range in HCM cases involving a limited number of LV segments (16). Apical HCM is the subtype most commonly missed on TTE due to acoustic window limitations and MRI has proven clinical utility in its diagnosis and characterization (17). Similarly an apical LV aneurysm associated with the mid-ventricular phenotype is often overlooked. An apical aneurysm appears as focal wall thinning and dyskinesia, often displaying transmural LGE and frequently containing mural thrombus (17). It is thought to result from chronically elevated mid-ventricular pressures and is important to diagnose as is associated with an adverse clinical course and annual event rate of 10.5% (18).
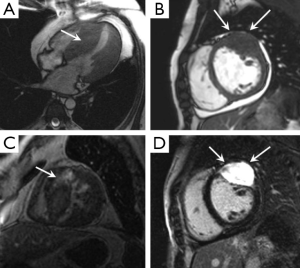
“Mass like” HCM can mimic the appearance of a myocardial based tumour, such as a fibroma. Key discriminating features on MRI are the presence of some residual contractility with HCM and typically a fibroma displays much more intense and often uniform LGE compared with HCM (Figure 2) (19). Right ventricular hypertrophy has been reported in 15-20% of HCM patients and most often involves the mid-to-apical portion of the RV, often contiguous with LVH. There are sporadic case reports of HCM causing right ventricular outflow tract obstruction (20).
Differential diagnosis for concentric LVH
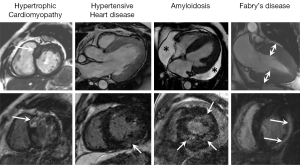
Concentric LVH accounts for approximately 10% of HCM cases and must be distinguished from other causes of concentric LVH which include hypertensive heart disease (HHD), aortic stenosis, sarcoidosis, amyloidosis, Anderson-Fabry disease and athletic remodeling (Figure 3). In many cases, the distinction from HCM related LVH is straightforward based on clinical features and key imaging determinates, namely, LVH distribution, LV function, and the presence and pattern of LGE but there can be a significant amount of overlap. For example myocardial fibrosis is a common end point of many myocardial diseases and Rudolph et al. (21) recently showed that 50% of patients with HHD had patchy mid-wall LGE, thought to reflect fibrosis-related expansion of the extracellular space which is a similar pattern to that seen with HCM (21,22). In general LV wall thickness of HHD is less pronounced than with HCM (typically no more than 15-16 mm). With aortic stenosis the valves leaflets appear thickened with restricted opening and typically there is a flow acceleration jet across the valve on cine SSFP sequences. Cardiac sarcoidosis typically shows LVH with LGE localized in the basal and subepicardial myocardium, often with signs of mediastinal lymphadenopathy and upper zone pulmonary fibrosis (23). Anderson-Fabry disease (X-linked lysosomal storage disorder) causes progressive myocardial accumulation of globotriaosylceramide leading to LVH and congestive cardiac failure. Recently LGE localized to the basal inferolateral wall has been described in this condition and is thought to be a pathognomonic MRI feature (24). Amyloid heart disease displays typical features of a restrictive cardiomyopathy with LVH, diastolic dysfunction, and bi-atrial enlargement. There is usually associated thickening of the interatrial septum and a pericardial effusion. The LGE pattern with amyloidosis is often distinctive owing to chelation of gadolinium within the interstitial space which produces a low blood pool signal. The classical LGE pattern is that of global subendocardial enhancement (25,26). Morphologic adaptations of an athlete’s heart typically cause mild LVH (usually <16 mm), increased left ventricular volumes but no evidence of diastolic dysfunction. A diastolic wall thickness divided by left ventricular end-diastolic volume ratio of less than 0.15 mm/m2/mL is regarded as the best parameter to differentiate an athlete’s heart from all other pathologic causes of LVH. Another important feature of athletic remodeling is a lack of LGE (27).
LVOT assessment
Resting or provocable LVOT obstruction is present in up to 70% of HCM cases and relates to complex anatomical relationships between the basal septum, LVOT, mitral valve, and papillary muscles (28). In the majority of HCM patients, septal hypertrophy leads directly to LVOT obstruction but LVOT obstruction can also occur in the presence of minimal septal thickening as the result of variant papillary muscle and subvalvular anatomy which highlights the importance of accurate anatomical assessment (29). Compared with control subjects, HCM patients have been shown to have a higher incidence of anomalous papillary muscles including bifid and accessory papillary muscles, as well as antero-apical papillary muscle displacement (Figure 4) (28,30). MRI is thought to have some advantages over TTE for anatomical LVOT assessment, especially with regards to the subvalvular anatomy and defining the precise point of septal-SAM contact (28). Although MRI can suggest the presence of a significant LVOT gradient (presence of flow acceleration) TTE remains the technique of choice for measurement of LVOT peak flow velocity owing to its higher temporal resolution and the ability to precisely align the TTE imaging plane to sensitively detect maximal systolic velocity. It is anticipated that newer MRI sequences in development, particularly those with ultra-short echo times may improve the reliability and accuracy of MRI assessment of the LVOT gradient in HCM (31). Four-dimensional (4D) phase contrast MRI may also hold promise for assessing the LVOT peak pressure gradient in HCM patients with Allen et al. recently describing higher grade nested helical flow patterns in obstructive HCM patients compared with non-obstructive HCM patients (P=0.04) and volunteers (P<0.001) (32).
Risk stratification
Although many HCM patients remain asymptomatic, SCD is a recognised disease manifestation with an estimated event rate of approximately 0.5% HCM patients per year (33). It is hypothesised that myocardial architectural disarray including myocyte hypertrophy and replacement fibrosis, create an unstable electrophysiological substrate and are important drivers of malignant arrhythmias (ventricular tachycardia and fibrillation) and SCD in HCM. A number of risk factors for SCD in HCM have emerged from large observational studies including non-sustained VT, unexplained recurrent syncope and a family history of HCM-related SCD (33). HCM patients deemed “high risk” may be offered placement of an implantable cardioverter-defibrillator (ICD) but decision making dilemmas often occur and there is limited guidance available on which to base referral for ICD implantation (34).
Recently there has been much interest in the role of MRI for risk assessment in HCM with several landmark papers confirming MRI derived fibrosis as an independent predictor of major adverse events and survival (35,36). Both the presence and severity of MRI LGE derived fibrosis assessment is associated with a greater risk of major adverse events with a positive correlation shown between the amount of LGE positivity and ventricular arrhythmias detected on ambulatory ECG recording. HCM patients with LGE positivity have been shown to have a sevenfold increased risk for lethal ventricular arrhythmias compared with those who are LGE negative (34). There is also emerging evidence that substantial LGE positivity in HCM patients with low clinical risk scores may be an independent adverse prognostic indicator (37). The decision to refer for ICD therapy for primary prevention based solely on LGE positivity alone is not currently advocated but in patients classified as intermediate risk of SCD based on traditional models LGE positivity is considered by many experts as a potential arbitrator for ICD decision making (34).
There is also a well-established relationship between the severity of left-ventricular hypertrophy and prognosis in patients with HCM. Specifically hypertrophy of ≥30 mm within any segment identifies HCM patients at the greatest risk of SCD. The presence of LVOT obstruction with a resting gradient >30 mmHg has also been shown as a strong predictor of SCD and may prompt referral for gradient reduction surgery such a myomectomy or catheter ablation (38).
Screening
Patients with pre-clinical HCM are those with genetic positivity but no LVH (phenotype negative). There is great interest in the early identification of HCM mutation carriers given the potential risk of SCD and other life threatening events (39). A number of TTE studies have shown abnormal diastolic dysfunction as an early marker of disease (40). MRI studies are few but several recent papers have described a high prevalence of myocardial crypts in genotype-positive/phenotype-negative HCM patients as a potential pre-phenotypic marker of HCM (41-43). Crypts are abrupt sharp-edged disruptions of normally compacted myocardium penetrating the left ventricular wall with varying depth (often more than a third of wall thickness) and show complete obliteration during systole (Figure 5) (41). They are not reliably detected with TTE and should not be confused with trabeculations, which are sponge-like networks of non-compacted myocardium. Maron et al. have reported MRI evidence of crypt formation in 61% of genotype-positive/phenotype-negative HCM patients (cohort derived from screening family members of known HCM) (41). Conversely, the prevalence of myocardial crypts was low (4%) in patients who are genotype/phenotype positive for HCM. These observations are supported by Deva et al. who have recently shown that basal inferoseptal crypts occur more commonly in patients with HCM with disease-causing mutations than in those with genotype-negative HCM (42).
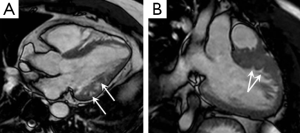
Myocardial crypt identification on MRI may have the potential to identify family members of HCM patients who are asymptomatic HCM mutation carriers, prompting close surveillance to monitor for development of the HCM phenotype (41-43).
Future directions
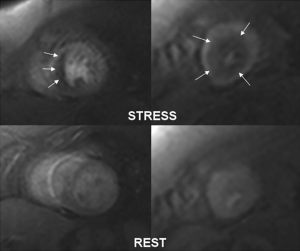
Standard LGE inversion recovery techniques are heavily reliant on operator determined “normal” nulled myocardial signal in order to identify diseased myocardium. As HCM is a diffuse myocardial disease only the most severely abnormal regions may be highlighted with LGE which may substantially underestimate global fibrosis burden (44). T1-mapping techniques have recently been developed which more sensitively detect myocardial fibrosis by amplification of all abnormal signal regions (44,45). T1-mapping holds particular promise in the setting of diffuse myocardial diseases such as HCM with the potential to more accurately quantify LGE volume which might hold particular prognostic relevance (45). Ischemia testing is another area which holds particular promise in HCM. Ismail at al. in a recent pixel-wise first pass adenosine MRI perfusion study identified a significant number of HCM patients with severe localized microvascular dysfunction associated with increasing wall thickness and with the presence of LGE (46).
Gyllenhammar et al. recently reported a cohort of young patients (age, 22.3±6.4 years) with HCM who showed reduced myocardial perfusion during adenosine induced hyperaemia compared with controls even in the absence of diastolic dysfunction or LV outflow obstruction (47). These results suggest that ischemia testing may reveal early arteriolar changes prior to development of replacement fibrosis and as such might be a novel marker of risk and even a potential therapeutic target (Figure 6) (48).
Conclusions
HCM is the most common genetic myocardial disorder and is characterized by a wide range of clinical and phenotypic expression. Non-invasive imaging is central to the diagnosis of HCM and MRI in particular is increasingly used to characterize abnormalities of morphology and tissue composition. In addition MRI has an emerging role in risk stratification and proband screening with future longitudinal studies expected to determine which MRI features are the most important in terms of disease progression and risk of sudden death.
Disclosure: The authors declare no conflict of interest.
References
- Maron BJ, Maron MS. Hypertrophic cardiomyopathy. Lancet 2013;381:242-55. [PubMed]
- Bos JM, Towbin JA, Ackerman MJ. Diagnostic, prognostic, and therapeutic implications of genetic testing for hypertrophic cardiomyopathy. J Am Coll Cardiol 2009;54:201-11. [PubMed]
- Hughes SE. The pathology of hypertrophic cardiomyopathy. Histopathology 2004;44:412-27. [PubMed]
- Charron P, Carrier L, Dubourg O, Tesson F, Desnos M, Richard P, Bonne G, Guicheney P, Hainque B, Bouhour JB, Mallet A, Feingold J, Schwartz K, Komajda M. Penetrance of familial hypertrophic cardiomyopathy. Genet Couns 1997;8:107-14. [PubMed]
- Nagueh SF, Bierig SM, Budoff MJ, Desai M, Dilsizian V, Eidem B, Goldstein SA, Hung J, Maron MS, Ommen SR, Woo A; American Society of Echocardiography; American Society of Nuclear Cardiology; Society for Cardiovascular Magnetic Resonance; Society of Cardiovascular Computed Tomography. American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with hypertrophic cardiomyopathy: Endorsed by the American Society of Nuclear Cardiology, Society for Cardiovascular Magnetic Resonance, and Society of Cardiovascular Computed Tomography. J Am Soc Echocardiogr 2011;24:473-98. [PubMed]
- Moon JC, Fisher NG, McKenna WJ, Pennell DJ. Detection of apical hypertrophic cardiomyopathy by cardiovascular magnetic resonance in patients with non-diagnostic echocardiography. Heart 2004;90:645-9. [PubMed]
- Rickers C, Wilke NM, Jerosch-Herold M, Casey SA, Panse P, Panse N, Weil J, Zenovich AG, Maron BJ. Utility of cardiac magnetic resonance imaging in the diagnosis of hypertrophic cardiomyopathy. Circulation 2005;112:855-61. [PubMed]
- Kramer CM, Barkhausen J, Flamm SD, Kim RJ, Nagel E; Society for Cardiovascular Magnetic Resonance Board of Trustees Task Force on Standardized Protocols. Standardized cardiovascular magnetic resonance imaging (CMR) protocols, society for cardiovascular magnetic resonance: board of trustees task force on standardized protocols. J Cardiovasc Magn Reson 2008;10:35. [PubMed]
- Choudhury L, Mahrholdt H, Wagner A, Choi KM, Elliott MD, Klocke FJ, Bonow RO, Judd RM, Kim RJ. Myocardial scarring in asymptomatic or mildly symptomatic patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 2002;40:2156-64. [PubMed]
- Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, Bundy J, Finn JP, Klocke FJ, Judd RM. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation 1999;100:1992-2002. [PubMed]
- Kitamura M, Shimizu M, Ino H, Okeie K, Yamaguchi M, Funjno N, Mabuchi H, Nakanishi I. Collagen remodeling and cardiac dysfunction in patients with hypertrophic cardiomyopathy: the significance of type III and VI collagens. Clin Cardiol 2001;24:325-9. [PubMed]
- Moon JC, Reed E, Sheppard MN, Elkington AG, Ho SY, Burke M, Petrou M, Pennell DJ. The histologic basis of late gadolinium enhancement cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol 2004;43:2260-4. [PubMed]
- Rubinshtein R, Glockner JF, Ommen SR, Araoz PA, Ackerman MJ, Sorajja P, Bos JM, Tajik AJ, Valeti US, Nishimura RA, Gersh BJ. Characteristics and clinical significance of late gadolinium enhancement by contrast-enhanced magnetic resonance imaging in patients with hypertrophic cardiomyopathy. Circ Heart Fail 2010;3:51-8. [PubMed]
- Moon JC, McKenna WJ, McCrohon JA, Elliott PM, Smith GC, Pennell DJ. Toward clinical risk assessment in hypertrophic cardiomyopathy with gadolinium cardiovascular magnetic resonance. J Am Coll Cardiol 2003;41:1561-7. [PubMed]
- Maron MS, Maron BJ, Harrigan C, Buros J, Gibson CM, Olivotto I, Biller L, Lesser JR, Udelson JE, Manning WJ, Appelbaum E. Hypertrophic cardiomyopathy phenotype revisited after 50 years with cardiovascular magnetic resonance. J Am Coll Cardiol 2009;54:220-8. [PubMed]
- Olivotto I, Maron MS, Autore C, Lesser JR, Rega L, Casolo G, De Santis M, Quarta G, Nistri S, Cecchi F, Salton CJ, Udelson JE, Manning WJ, Maron BJ. Assessment and significance of left ventricular mass by cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol 2008;52:559-66. [PubMed]
- Kebed KY, Al Adham RI, Bishu K, Askew JW, Klarich KW, Araoz PA, Foley TA, Glockner JF, Nishimura RA, Anavekar NS. Evaluation of apical subtype of hypertrophic cardiomyopathy using cardiac magnetic resonance imaging with gadolinium enhancement. Am J Cardiol 2014;114:777-82. [PubMed]
- Maron MS, Finley JJ, Bos JM, Hauser TH, Manning WJ, Haas TS, Lesser JR, Udelson JE, Ackerman MJ, Maron BJ. Prevalence, clinical significance, and natural history of left ventricular apical aneurysms in hypertrophic cardiomyopathy. Circulation 2008;118:1541-9. [PubMed]
- Sparrow PJ, Kurian JB, Jones TR, Sivananthan MU. MR imaging of cardiac tumors. Radiographics 2005;25:1255-76. [PubMed]
- Hansen MW, Merchant N. MRI of hypertrophic cardiomyopathy: part I, MRI appearances. AJR Am J Roentgenol 2007;189:1335-43. [PubMed]
- Rudolph A, Abdel-Aty H, Bohl S, Boyé P, Zagrosek A, Dietz R, Schulz-Menger J. Noninvasive detection of fibrosis applying contrast-enhanced cardiac magnetic resonance in different forms of left ventricular hypertrophy relation to remodeling. J Am Coll Cardiol 2009;53:284-91. [PubMed]
- Ho CY, López B, Coelho-Filho OR, Lakdawala NK, Cirino AL, Jarolim P, Kwong R, González A, Colan SD, Seidman JG, Díez J, Seidman CE. Myocardial fibrosis as an early manifestation of hypertrophic cardiomyopathy. N Engl J Med 2010;363:552-63. [PubMed]
- Smedema JP, Snoep G, van Kroonenburgh MP, van Geuns RJ, Dassen WR, Gorgels AP, Crijns HJ. Evaluation of the accuracy of gadolinium-enhanced cardiovascular magnetic resonance in the diagnosis of cardiac sarcoidosis. J Am Coll Cardiol 2005;45:1683-90. [PubMed]
- Moon JC, Sachdev B, Elkington AG, McKenna WJ, Mehta A, Pennell DJ, Leed PJ, Elliott PM. Gadolinium enhanced cardiovascular magnetic resonance in Anderson-Fabry disease. Evidence for a disease specific abnormality of the myocardial interstitium. Eur Heart J 2003;24:2151-5. [PubMed]
- Falk RH, Dubrey SW. Amyloid heart disease. Prog Cardiovasc Dis 2010;52:347-61. [PubMed]
- Maceira AM, Joshi J, Prasad SK, Moon JC, Perugini E, Harding I, Sheppard MN, Poole-Wilson PA, Hawkins PN, Pennell DJ. Cardiovascular magnetic resonance in cardiac amyloidosis. Circulation 2005;111:186-93. [PubMed]
- Petersen SE, Selvanayagam JB, Francis JM, Myerson SG, Wiesmann F, Robson MD, Ostman-Smith I, Casadei B, Watkins H, Neubauer S. Differentiation of athlete's heart from pathological forms of cardiac hypertrophy by means of geometric indices derived from cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2005;7:551-8. [PubMed]
- Maron MS, Olivotto I, Harrigan C, Appelbaum E, Gibson CM, Lesser JR, Haas TS, Udelson JE, Manning WJ, Maron BJ. Mitral valve abnormalities identified by cardiovascular magnetic resonance represent a primary phenotypic expression of hypertrophic cardiomyopathy. Circulation 2011;124:40-7. [PubMed]
- Schulz-Menger J, Abdel-Aty H, Busjahn A, Wassmuth R, Pilz B, Dietz R, Friedrich MG. Left ventricular outflow tract planimetry by cardiovascular magnetic resonance differentiates obstructive from non-obstructive hypertrophic cardiomyopathy. J Cardiovasc Magn Reson 2006;8:741-6. [PubMed]
- Kwon DH, Setser RM, Thamilarasan M, Popovic ZV, Smedira NG, Schoenhagen P, Garcia MJ, Lever HM, Desai MY. Abnormal papillary muscle morphology is independently associated with increased left ventricular outflow tract obstruction in hypertrophic cardiomyopathy. Heart 2008;94:1295-301. [PubMed]
- O’Brien KR, Myerson SG, Cowan BR, Young AA, Robson MD. Phase contrast ultrashort TE: A more reliable technique for measurement of high-velocity turbulent stenotic jets. Magn Reson Med 2009;62:626-36. [PubMed]
- Allen BD, Choudhury L, Barker AJ, van Ooij P, Collins JD, Bonow RO, Carr JC, Markl M. Three-dimensional haemodynamics in patients with obstructive and non-obstructive hypertrophic cardiomyopathy assessed by cardiac magnetic resonance. Eur Heart J Cardiovasc Imaging. 2014. pii: jeu146. [Epub ahead of print].
- Elliott PM, Poloniecki J, Dickie S, Sharma S, Monserrat L, Varnava A, Mahon NG, McKenna WJ. Sudden death in hypertrophic cardiomyopathy: identification of high risk patients. J Am Coll Cardiol 2000;36:2212-8. [PubMed]
- Maron BJ, Spirito P. Implantable defibrillators and prevention of sudden death in hypertrophic cardiomyopathy. J Cardiovasc Electrophysiol 2008;19:1118-26. [PubMed]
- Kwon DH, Smedira NG, Rodriguez ER, Tan C, Setser R, Thamilarasan M, Lytle BW, Lever HM, Desai MY. Cardiac magnetic resonance detection of myocardial scarring in hypertrophic cardiomyopathy: correlation with histopathology and prevalence of ventricular tachycardia. J Am Coll Cardiol 2009;54:242-9. [PubMed]
- Maron MS, Appelbaum E, Harrigan CJ, Buros J, Gibson CM, Hanna C, Lesser JR, Udelson JE, Manning WJ, Maron BJ. Clinical profile and significance of delayed enhancement in hypertrophic cardiomyopathy. Circ Heart Fail 2008;1:184-91. [PubMed]
- Chan RH, Maron BJ, Olivotto I, Pencina MJ, Assenza GE, Haas T, Lesser JR, Gruner C, Crean AM, Rakowski H, Udelson JE, Rowin E, Lombardi M, Cecchi F, Tomberli B, Spirito P, Formisano F, Biagini E, Rapezzi C, De Cecco CN, Autore C, Cook EF, Hong SN, Gibson CM, Manning WJ, Appelbaum E, Maron MS. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation 2014;130:484-95. [PubMed]
- Nishimura RA, Holmes DR Jr. Clinical practice. Hypertrophic obstructive cardiomyopathy. N Engl J Med 2004;350:1320-7. [PubMed]
- Seidman JG, Seidman C. The genetic basis for cardiomyopathy: from mutation identification to mechanistic paradigms. Cell 2001;104:557-67. [PubMed]
- Ho CY, Sweitzer NK, McDonough B, Maron BJ, Casey SA, Seidman JG, Seidman CE, Solomon SD. Assessment of diastolic function with Doppler tissue imaging to predict genotype in preclinical hypertrophic cardiomyopathy. Circulation 2002;105:2992-7. [PubMed]
- Maron MS, Rowin EJ, Lin D, Appelbaum E, Chan RH, Gibson CM, Lesser JR, Lindberg J, Haas TS, Udelson JE, Manning WJ, Maron BJ. Prevalence and clinical profile of myocardial crypts in hypertrophic cardiomyopathy. Circ Cardiovasc Imaging 2012;5:441-7. [PubMed]
- Deva DP, Williams LK, Care M, Siminovitch KA, Moshonov H, Wintersperger BJ, Rakowski H, Crean AM. Deep basal inferoseptal crypts occur more commonly in patients with hypertrophic cardiomyopathy due to disease-causing myofilament mutations. Radiology 2013;269:68-76. [PubMed]
- Moon JC, McKenna WJ. Myocardial crypts: a prephenotypic marker of hypertrophic cardiomyopathy? Circ Cardiovasc Imaging 2012;5:431-2. [PubMed]
- White SK, Piechnik SK, Neubauer S, Robson MD, Moon J. Histological validation of ShMOLLI equilibrium contrast CMR for the measurement of diffuse myocardial fibrosis. J Cardiovasc Magn Reson 2012;14:O111.
- Brouwer WP, Baars EN, Germans T, de Boer K, Beek AM, van der Velden J, van Rossum AC, Hofman MB. In-vivo T1 cardiovascular magnetic resonance study of diffuse myocardial fibrosis in hypertrophic cardiomyopathy. J Cardiovasc Magn Reson 2014;16:28. [PubMed]
- Ismail TF, Hsu LY, Greve AM, Gonçalves C, Jabbour A, Gulati A, Hewins B, Mistry N, Wage R, Roughton M, Ferreira PF, Gatehouse P, Firmin D, O’Hanlon R, Pennell DJ, Prasad SK, Arai AE. Coronary microvascular ischemia in hypertrophic cardiomyopathy - a pixel-wise quantitative cardiovascular magnetic resonance perfusion study. J Cardiovasc Magn Reson 2014;16:49. [PubMed]
- Gyllenhammar T, Fernlund E, Jablonowski R, Jögi J, Engblom H, Liuba P, Arheden H, Carlsson M. Young patients with hypertrophic cardiomyopathy, but not subjects at risk, show decreased myocardial perfusion reserve quantified with CMR. Eur Heart J Cardiovasc Imaging 2014. pii: jeu137. [Epub ahead of print].
- Maron MS, Olivotto I, Maron BJ, Prasad SK, Cecchi F, Udelson JE, Camici PG. The case for myocardial ischemia in hypertrophic cardiomyopathy. J Am Coll Cardiol 2009;54:866-75. [PubMed]


