Age-related fatty infiltration of lumbar paraspinal muscles: a normative reference database study in 516 Chinese females
Introduction
Atrophy and fatty infiltration of muscle with aging are associated with sarcopenia, obesity, and osteoporosis (1-4). The muscle imaging assessment is of increasing interest in musculoskeletal research, especially in sarcopenia. Sarcopenia is an aging-related condition, defined by a progressive and generalized loss of muscle mass and function (5,6). Two commonly used techniques to assess lean and fat mass are dual X-ray absorptiometry (DXA) and bioelectrical impedance (BIA). However, neither of them provides a spatial distribution of muscle and adipose tissue. Fatty infiltration, as a result of the aging process, has been shown as an essential biomarker of muscle degeneration (7,8). The advantage of using CT (and MRI) over DXA or BIA in related investigations is the ability to assess fatty infiltration. Thus, CT or MRI will be critical techniques to address the pathophysiology of sarcopenia (9,10).
The relationship between trunk muscle, particularly paraspinal muscle deficits, and disc degeneration disease of the lumbar spine, along with kyphosis, had raised considerable attention (11-13). Moreover, lumbar muscle degeneration is a common feature in low back pain. Visibly, this muscle degeneration is characterized by a decreased cross-sectional area (muscle atrophy) and an increase in fat content (fatty infiltration) in the lumbar paraspinal muscles (14). Although changes in muscle size and fat infiltration of the lumbar muscles are frequently reported in low back pain literature, age-related physiological changes of paraspinal muscle were not well studied. The lack of references related to structural changes makes it difficult to draw abnormal identification. Multiple published reports establish the use of CT and MRI in assessing paraspinal muscle degeneration. CT scanning shows this phenomenon by density or size, while MRI detects paraspinal muscle degeneration by muscle properties (15-21). However, most of these studies were retrospectively based on images of patients with chronic low back pain, and little is known about atrophy and fatty infiltration of paraspinal muscle in a healthy population.
Furthermore, paraspinal muscle aging within the Chinese population is not well studied, despite China having the largest population in the world, which is also rapidly aging. To our knowledge, age-related reference data of atrophy and fatty infiltration of paraspinal muscles during adulthood of healthy Chinese females have not been reported. The diagnostic cutoff values for pathologic paraspinal muscle atrophy in the Chinese population may differ from Caucasians due to genetic factors, environmental conditions, body size, and lifestyles. The purpose of this study was to: (I) assess the age-related differences in paraspinal muscle in healthy Chinese females aged 20–80 years; (II) provide reference data of paraspinal muscle and visible deposit of adipose tissue; (III) explore how body size affects fatty infiltration of paraspinal muscle.
Methods
Subjects
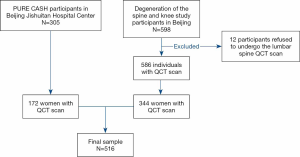
Five hundred and sixteen subjects (21 to 79 years old) in this cross-sectional study originated from two cohorts. One cohort comprised of 344 healthy women and was recruited from an ongoing study that has been running since June 2014. This ongoing study examined the degeneration of the spine and knee, described previously (22). The second cohort comprised of one hundred and seventy-two women from the Prospective Urban Rural Epidemiology (PURE) China Action on Spine and Hip status (CASH) study (23,24). All women were living independently in the community, with details of eligibility and recruitment previously published (22,24). In brief, for the degeneration of the spine and knee study, the criteria for inclusion were healthy adults, aged 20–65 years, and a resident of Beijing >5 years; for the PURE CASH study, the inclusion criteria were healthy adults aged over 40 years. This study included study subjects from Beijing Jishuitan Hospital. Participants with previous and current use of hormones were excluded. Additional criteria for exclusion were a severe organic disease, systemic metabolic bone disease, lipodystrophy, and recent history of lumbar vertebrae fracture and surgery. A flowchart detailing stepwise inclusion and exclusion of the subjects is presented in Figure 1. We have obtained ethical approval from the ethics committee of the Beijing Jishuitan Hospital (Approval No. 201210-01 and No. 201512-02). The study is conducted following ethical principles according to the Declaration of Helsinki and is consistent with Good Clinical Practice. Radiation safety and protection measures are strictly implemented in the whole study. Informed consent was obtained from all participants in the study.
Anthropometry
Height (nearest 0.1 cm) and bodyweight (nearest 0.01 kg) were measured with a standard height and weight scale. BMI was calculated using formula: Weight/Height2 (kg/m2). A BMI <24 kg/m2 was considered normal; 24–28 kg/m2 was considered overweight; >28 kg/m2 was defined obesity based on the Chinese criteria (25).
QCT scans
Spine QCT scans of the cohort were acquired from a Toshiba Aquilion 80-slices CT scanner (Toshiba Medical Systems, Tokyo, Japan) together with a Mindways calibration phantom (Mindways Software Inc., Austin, TX, USA). The lumbar were scanned in the supine position from L1 to L5. Acquired scan parameters included exposure factors of 120 kVp, 187 mAs, 1-mm slice thickness, and 40 cm field of view (DFOV), with standard reconstruction.
Measurements of paraspinal muscles and fat
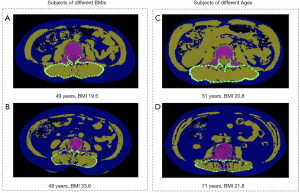
Images were transferred to a QCT Pro workstation (Mindways Software, Version 4.2.3; Mindways, Austin, TX, USA) by the image transfer utility set up on the CT scanner. We measured the cross-section area (CSA) of paraspinal muscles, including multifidus muscle and erector spinae muscle, and intermuscular adipose tissue (IMAT) at the mid-vertebral level slice of L3. In our study, IMAT was defined as an ectopic fat deposit beneath the fascia and within the muscles following Addison’s review (26). Simultaneously, measurements of CSA of paraspinal muscles (CSAmuscle) and IMAT (CSAIMAT) were both semi-automatically completed by the commercial software package: “Tissue Composition Module” Beta 1.0 (Mindways, Austin, TX, USA). The QCT Pro Tissue Composition Module could define an optional contour around a muscle or group of muscles. A spline was used to constrain the location of the contour, and a “snake” operation fitted the contour precisely to the muscle group (Figure 2). The resulting spline boundary can be modified by the user, as needed, by moving the spline control points. In contrast to absolute HU values based on CT images in the literature (14), HU measurements based on QCT imaging are converted into tissue (i.e., Muscle and fat) densities by scanning the subject together with a calibration phantom containing standards representing known densities of bone and soft tissue. In this study, the Tissue Composition Module of QCT automatically selected the water (H2O) basis set equivalent density as segmentation thresholds based on calibration data from CT images. The density thresholds used for fat segmentation were 833 to 995 mg/cm3 H2O. Segmentation thresholds for muscle were 996 to 1,153 mg/cm3 H2O. Subsequently, IMAT% (CSAIMAT / CSAIMAT + CSAmuscle) indicating fatty infiltration of muscle were calculated, based on the measurements of muscle CSA and IMAT CSA. The intra-observer reproducibility of the measurements for fat and paraspinal muscles CSA of L3 was high, with intraclass correlation coefficients of 0.996 and 0.993, respectively. The inter-class correlation coefficients of measurements for fat and muscle CSA were 0.995 and 0.981.
Statistical analysis
Data were expressed as mean ± SD. Kruskal-Wallis H test was used to compare the differences in anthropometric characteristics and measurement results among age groups by decade. Kruskal-Wallis H test and LSD post-hoc correction was used to compare the results among BMI subgroups in young (20–39 years), middle-aged (40–59 years), and elderly (60–79 years) groups, respectively. The relationships between age, height, weight, BMI, and measurements of paraspinal muscles and IMAT were evaluated using Spearman correlations, depending on the distribution of the variables. Correlation coefficient r between 1.0 and 0.5 was considered strong; 0.5–0.3, moderate; and 0.3–0.1, weak. We used the Lowess curve fitting to determine if there was a linear or nonlinear correlation between age and paraspinal muscles and IMAT measurements. Analysis of covariance was used to calculate the interaction of age and BMI for parameters. Changes in measurement results with age, by decade, were calculated using height and weight as covariate variables. Statistical analyses were performed by using commercial software (SPSS, Version 25 for Mac; IBM, Armonk, New York, USA). P<0.05 was considered statistically significant.
Results
Anthropometric characteristics and measurements of paraspinal muscles and IMAT
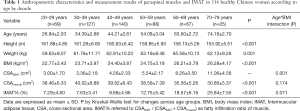
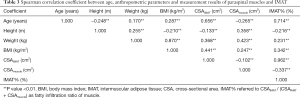
The mean age of 516 healthy Chinese females was 45.12±13.41 years (range, 21–79 years). The anthropometric parameters and measurements of paraspinal muscles and fat among age groups by decade are provided in Table 1. Interaction between age and BMI was significant for CSAIMAT. Significant differences in height, weight, BMI, CSAmuscle, and IMAT% were found among age groups by decade.
Full table
The effect of body size on CSAIMAT, CSAmuscle and IMAT% in different age groups
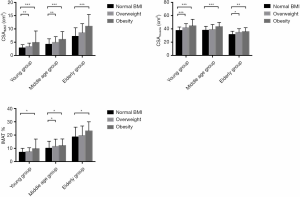
Table 2 and Figure 3 show measurement results among BMI subgroups in young (20–39 years), middle-aged (40–59 years), and elderly (60–79 years) groups. All age groups displayed significant differences between obesity and normal BMI subgroups for CSAIMAT and CSAmuscle. IMAT% showed similar trends in all age groups. The young and middle-aged group showed significant differences for CSAIMAT across overweight and normal BMI subgroups.

Full table
Correlation of measurement variables with age and anthropometric parameters
Lowess curve fitting indicated a nonlinear correlation between CSAIMAT, IMAT%, and age. However, the association between CSAmuscle and age was linear (Figure 4). CSAIMAT, IMAT% increased with aging, whereas CSAmuscle decreased with increasing age.
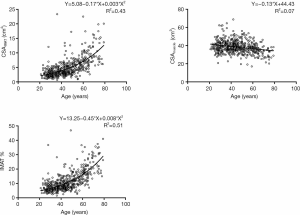
Correlation coefficients of measurement variables with age and anthropometric parameters are presented in Table 3. Strong positive associations of age with CSAIMAT and IMAT% were shown (r=0.656, P<0.01; r=0.714, P<0.01). A weak negative association between CSAmuscle and age was observed (r=−0.265, P<0.01). We also found positive relations of BMI with CSAIMAT and CSAmuscle (r=0.441, P<0.01; r=0.247, P<0.01) (Table 3). Interestingly, CSAIMAT was negatively associated with CSAmuscle (r=−0.102, P<0.01).
Full table
Age-related differences in CSAIMAT, CSAmuscle, and IMAT%
Table 4 lists the percentage change of measurements, adjusted for height and weight per decade over the age range of 21–79 years. Compared to muscle, CSAIMAT showed a larger age-related difference (the percentage change of 20 to 79 years for CSAIMAT +212.5% vs. CSAmuscle −17.1%). CSAmuscle showed a comparatively mild decrease, demonstrating a change of less than 20% between the ages of 20 and 79 years. Subjects aged between 20 and 39 years, showed only a 2.0% increase for CSAIMAT and a slight 2.7% increase for CSAmuscle across a decade increase in age. Between 60–79 years, the percent change of CSAIMAT and CSAmuscle per decade were prominently greater than that of the younger age ranges. CSAIMAT also revealed significant change between 40–59 years compared with the 20–39 years group. The changes in IMAT% showed a similar trend to that observed with CSAIMAT.

Full table
Discussion
In this study, we applied QCT imaging to quantify paraspinal muscles (multifidus and erect spinae muscle) and intermuscular adipose tissue at the 3rd lumbar vertebrae. This method was used to investigate age-related changes in fatty degeneration in paraspinal muscles. A total of 516 healthy Chinese women (21–79 years of age) took part in this study. Our results indicated that fatty infiltration in paraspinal muscles increased with age, while muscle loss might be associated with aging. We also found that fatty infiltration in paraspinal muscles increased with BMI in different age groups.
Our study showed that the fat infiltration ratio of combined multifidus and erector spinae muscle was 9.68%±4.96% for 40–49 years group and 25.84%±7.55% for 70–79 years group. A similar measurement conducted with MRI in a Japanese female population demonstrated the fat infiltration ratio of separated erector spinae muscle at L3/L4 level was 6.7%±1.5% for <50 years group and 12.4%±3.8% for 70–79 years group. The same study also revealed the infiltration ratio of separated multifidus muscle was 6.5%±1.9% for <50 years group and 13.9%±5.4% for 70–79 years group (15). The inconsistency of fat filtration ratio values in the 70–79 age group between our findings and the trial above is unclear. However, according to Kalichman’s paraspinal muscle scoring system (27) (grade 1, a normal muscle condition, fatty infiltration up to 10% of the muscle’s CSA; grade 2, moderate muscle degeneration, 10–50% of fatty infiltration; and grade 3, severe muscle degeneration, >50% of fatty infiltration), a fat infiltration ratio of about 12% for Japanese 70–79 years group seems low. Another longitudinal study revealed the highest percentage of fat in multifidus muscle, measured by MRI at L4 and L5 level, was 28.8%±12.7% for baseline 40 years of age, 28.7%±11.9% for 45 years at follow up and 31.6%±13.0% for 49 years in Danish population (28). Compared to our study, the higher fatty infiltration of the multifidus muscle in Danish may be due to differences in ethnicity, detection methods, measurement sites, and target muscle. A previous study found quantifying differences in paraspinal muscle CSA between MRI and CT measurement (13). Previous studies showed that fatty degeneration of paraspinal muscles was more prominent in the lower lumbar level than the upper lumbar level (13,29). Furthermore, the target muscle also affects the interpretation of the study results. The multifidus muscle has two fiber directions; vertical and horizontal to the spinal axis, which can disperse the stress. On the contrary, the erector spinae muscles are mainly vertical to the spinal axis, bearing more stress than multifidus muscle. As such, the multifidus muscle displayed more fatty degeneration than the erector spinae muscles (29).
Concerning the degeneration of paraspinal muscles, most previous studies focused on the associations between paraspinal muscle size and low back pain, kyphosis, or osteoporosis fracture (3,17-20,30). Several studies mainly focused on applying the paraspinal muscle composition assessment to the elderly population (19,20,30). However, its application to young and middle-aged populations are not widely investigated. Only a few publications have evaluated muscle sizes and fatty infiltration of paraspinal muscles across the lifespan of healthy populations (29,31,32). However, no studies have reported on the reference data for paraspinal muscle degeneration of healthy people in China. CT and MRI are the gold standards for estimating muscle mass among numerous measuring techniques applied in research and routine clinical practice (5). The majority of the above studies used MRI to measure muscle and fat CSA. To the best of our knowledge, our study is the first to present normative reference values of those parameters in healthy Chinese females.
Similar to previous studies, the paraspinal muscle size tended to decrease with age, while the fat infiltration rate increased with age (16,21,29). However, Crawford et al. reported a different trend in age-related muscle CSA change, where multifidus and erector spinae volume was age-independent in 80 healthy adult volunteers using MRI (31). The inconsistency of results between studies may be related to methodologic differences in the measurement methods (CT versus MRI) and parameters (CSA versus volume), targeted muscle groups, and study sample population.
In this study, we used a QCT-based method for the paraspinal muscles and infiltrated fat measurements, rather than HUs (Hounsfield Units). This is because HUs for segmentation and the measurement of muscle and infiltrated adipose tissue vary significantly across different studies (14). For muscle segmentation, different HU thresholds used in literature may not be very relevant, as in semi-automatic measurement procedures, initial threshold-based contours guide the operator and could be corrected if necessary. Compared to segmentation, differences in HUs have a significant impact on the quantification of adipose tissue found within the perimuscular deep fascia, such as the paraspinal muscles. The concern with using absolute CT thresholds stems from unaccounted deviations from the ideal water calibration of the CT scanner. The thresholds are also inconsistent across the literature (14,33).
For this reason, the separation of intermuscular and intramuscular adipose tissue components is of interest. Some studies have suggested techniques based on in-scan calibration phantoms (34,35). In this study, all CT scans were acquired with a QCT calibration phantom. The calculation of the paraspinal muscle and infiltrated adipose tissue was based on the CT calibration data, derived from the phantom scanned simultaneously with subject imaging. However, the sensitivity of the calibration method relative to the threshold selection has not been reported and needs further studies. Another issue is that the cross-sectional CT imaging would be affected by partial volume effects in measurements of muscle and adipose tissue. Thicker slices reduce noise and are adequate when the muscle volume or density is of primary interest. For a more advanced analysis of the adipose tissue distribution, a higher spatial resolution is required, and a slice thickness of 2 mm is preferable (14).
Expressed as absolute change between 20–79 years, or decade percentage change, CSAIMAT both showed more significant age-related differences than CSAmuscle. Notably, CSAIMAT was negatively associated with CSAmuscle, which indicates that muscle infiltration might partly cause muscle loss. This phenomenon requires further investigation. A study conducted by Dorien reported a higher amount of fat infiltration in the lumbar muscles without differences in muscle CSA in adults with continuous chronic back pain, compared to recurrent low back pain (17). Fat infiltration of skeletal muscle, either solely or along with muscle mass loss, appears to contribute to age-related depletion in skeletal muscle function.
Our results demonstrated that people over 40 years showed more prominent changes with age for CSA of IMAT and fatty infiltration. The percentage change per decade identified 40 years as the potential age, where a significant change in paraspinal muscle composition occurs across the life span of Chinese females. The peak of muscle mass is achieved in early adult life, followed by a progressive decline after the age of 40 (10,36). After the age of 50, the decline of muscle mass becomes more substantial, proceeding at a rate of less than 3% per year (10,37). However, the available data was mostly based on whole-body DXA measurements, which are mostly different from the assessment tools in the study. It is noteworthy that there is no data available on validated measurements of muscle health and its relationship with gender and ethnicity across adulthood. Our study demonstrates cross-sectional age-related changes in paraspinal muscle in females aged 20 to 79 years. These changes were examined on a per-decade basis. The study indicates the commencement of lumbar muscle decline in females in the 4th decade. These results are earlier than other reports in the literature, where muscle decline occurred after the age of 50 years. Little is known about normative degeneration of the lumbar paraspinal muscles. Most paraspinal muscle relevant studies examine patients with low back pain or severe spine degeneration. Our observation would contribute to the difficulty in estimating the age-related decline in skeletal muscle in adulthood due to incomplete data sets across age ranges. However, the findings of this study require validation with additional cohorts and further studies.
In our study, obesity groups revealed significantly greater fatty infiltration, compared to normal BMI groups across adulthood, while positive relationships were observed between BMI with CSAIMAT and CSAmuscle. A clear correlation between obese and overweight subgroups with higher CSAmuscle (Figure 3) is consistent with previous reports, where obese and overweight adults often have a higher muscle mass compared to their non-obese peers (38,39). These findings may be due to the extra loading of adipose tissue seen in both subgroups, acting as a training stimulus on skeletal muscle (40). However, our findings demonstrated that body size has a more significant influence on intermuscular fat deposition than increasing muscle mass. Primarily, a higher BMI (often defined as more obese) would introduce more significant muscle fatty infiltration.
In summary, the consensus is that obese adolescents exhibit lower relative muscle quality, despite displaying a larger muscle size. Valentin et al. examined bilateral lumbar erector spinae, multifidus, rectus abdominis, and psoas muscles of twenty-four healthy adults using T1-weighted axial MRI. They reported age and BMI as relevant factors for paraspinal extensor muscle volume (32). In contrast, Crawford et al. showed no significant correlation between BMI and fat signal fraction in multifidus and erector spinae (31). The discrepancy among these studies may be due to methodological differences.
There are several limitations to our study. First, study subjects in this study were exposed to radiation from CT scans to measure the fatty infiltration of paraspinal muscles. However, the CT data used in the current study were obtained from the QCT results of an ongoing spine and knee degeneration and the CASH study. Therefore, the study participants were not subjected to additional radiation exposure. Second, the CSA measurements of paraspinal muscles and IMAT were isolated to the L3 vertebral level, instead of analyzing the whole volume of paraspinal muscles. Third, only multifidus muscle and erector spinae muscle were examined, excluding psoas major muscle in this study. Fourth, data from the male population was not examined. Although more than 500 participants were included in the present analysis, the study population was recruited exclusively from Beijing. Therefore, the data may not be representative of the general female population, as it does not account for women of rural areas or other ethnic populations.
Furthermore, the body height of the study population was lower than the average value of female residents in Beijing. These findings were particularly observed in the young group. These results may be due to a selection bias, which would have affected data interpretation. Last, the fatty infiltration of the paraspinal muscles would be affected by the lumbar degeneration. However, as participants in this study were healthy and without severe degeneration, we consider the study population as representative of the respective age groups, demonstrating age-related physiological changes.
In conclusion, our study provides a normal reference data of fatty degeneration of paraspinal muscles for healthy, Chinese adult women. Future studies are required to investigate changes of paraspinal muscle morphology and composition with clinical implications for physical function and mortality.
Acknowledgments
Funding: This study was funded by the Training Program of the Major Research Plan of the National Natural Science Foundation of China (91959117).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Giuseppe Guglielmi and Alberto Bazzocchi) for the special issue “Body Composition Imaging” published in Quantitative Imaging in Medicine and Surgery. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/qims-19-835). The special issue “Body Composition Imaging” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board of Beijing Jishuitan Hospital. Informed consent was obtained from each subject.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Buch A, Carmeli E, Boker LK, Marcus Y, Shefer G, Kis O, Berner Y, Stern N. Muscle function and fat content in relation to sarcopenia, obesity and frailty of old age--An overview. Exp Gerontol 2016;76:25-32. [Crossref] [PubMed]
- Choi KM. Sarcopenia and sarcopenic obesity. Korean J Intern Med 2016;31:1054-60. [Crossref] [PubMed]
- Sheu Y, Marshall LM, Holton KF, Caserotti P, Boudreau RM, Strotmeyer ES, Cawthon PM, Cauley JA. Abdominal body composition measured by quantitative computed tomography and risk of non-spine fractures: the Osteoporotic Fractures in Men (MrOS) Study. Osteoporos Int 2013;24:2231-41. [Crossref] [PubMed]
- Yoshimura N, Muraki S, Oka H, Iidaka T, Kodama R, Kawaguchi H, Nakamura K, Tanaka S, Akune T. Is osteoporosis a predictor for future sarcopenia or vice versa? Four-year observations between the second and third ROAD study surveys. Osteoporos Int 2017;28:189-99. [Crossref] [PubMed]
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinkova E, Vandewoude M, Zamboni M. European Working Group on Sarcopenia in Older P. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412-23. [Crossref] [PubMed]
- Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, Chou MY, Chen LY, Hsu PS, Krairit O, Lee JS, Lee WJ, Lee Y, Liang CK, Limpawattana P, Lin CS, Peng LN, Satake S, Suzuki T, Won CW, Wu CH, Wu SN, Zhang T, Zeng P, Akishita M, Arai H. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 2014;15:95-101. [Crossref] [PubMed]
- Borkan GA, Hults DE, Gerzof SG, Robbins AH, Silbert CK. Age changes in body composition revealed by computed tomography. J Gerontol 1983;38:673-7. [Crossref] [PubMed]
- Marcus RL, Addison O, Kidde JP, Dibble LE, Lastayo PC. Skeletal muscle fat infiltration: impact of age, inactivity, and exercise. J Nutr Health Aging 2010;14:362-6. [Crossref] [PubMed]
- Messina C, Maffi G, Vitale JA, Ulivieri FM, Guglielmi G, Sconfienza LM. Diagnostic imaging of osteoporosis and sarcopenia: a narrative review. Quant Imaging Med Surg 2018;8:86-99. [Crossref] [PubMed]
- Guerri S, Mercatelli D, Aparisi Gomez MP, Napoli A, Battista G, Guglielmi G, Bazzocchi A. Quantitative imaging techniques for the assessment of osteoporosis and sarcopenia. Quant Imaging Med Surg 2018;8:60-85. [Crossref] [PubMed]
- Shahidi B, Parra CL, Berry DB, Hubbard JC, Gombatto S, Zlomislic V, Allen RT, Hughes-Austin J, Garfin S, Ward SR. Contribution of Lumbar Spine Pathology and Age to Paraspinal Muscle Size and Fatty Infiltration. Spine (Phila Pa 1976) 2017;42:616-23. [Crossref] [PubMed]
- Shahidi B, Hubbard JC, Gibbons MC, Ruoss S, Zlomislic V, Allen RT, Garfin SR, Ward SR. Lumbar multifidus muscle degenerates in individuals with chronic degenerative lumbar spine pathology. J Orthop Res 2017;35:2700-6. [Crossref] [PubMed]
- Hyun SJ, Bae CW, Lee SH, Rhim SC. Fatty Degeneration of the Paraspinal Muscle in Patients With Degenerative Lumbar Kyphosis: A New Evaluation Method of Quantitative Digital Analysis Using MRI and CT Scan. Clin Spine Surg 2016;29:441-7. [Crossref] [PubMed]
- Engelke K, Museyko O, Wang L, Laredo JD. Quantitative analysis of skeletal muscle by computed tomography imaging-State of the art. J Orthop Translat 2018;15:91-103. [Crossref] [PubMed]
- Sasaki T, Yoshimura N, Hashizume H, Yamada H, Oka H, Matsudaira K, Iwahashi H, Shinto K, Ishimoto Y, Nagata K, Teraguchi M, Kagotani R, Muraki S, Akune T, Tanaka S, Kawaguchi H, Nakamura K, Minamide A, Nakagawa Y, Yoshida M. MRI-defined paraspinal muscle morphology in Japanese population: The Wakayama Spine Study. PLoS One 2017;12:e0187765. [Crossref] [PubMed]
- Johannesdottir F, Allaire B, Anderson DE, Samelson EJ, Kiel DP, Bouxsein ML. Population-based study of age- and sex-related differences in muscle density and size in thoracic and lumbar spine: the Framingham study. Osteoporos Int 2018;29:1569-80. [Crossref] [PubMed]
- Goubert D, De Pauw R, Meeus M, Willems T, Cagnie B, Schouppe S, Van Oosterwijck J, Dhondt E, Danneels L. Lumbar muscle structure and function in chronic versus recurrent low back pain: a cross-sectional study. Spine J 2017;17:1285-96. [Crossref] [PubMed]
- Wan Q, Lin C, Li X, Zeng W, Ma C. MRI assessment of paraspinal muscles in patients with acute and chronic unilateral low back pain. Br J Radiol 2015;88:20140546. [Crossref] [PubMed]
- Fortin M, Gibbons LE, Videman T, Battie MC. Do variations in paraspinal muscle morphology and composition predict low back pain in men? Scand J Med Sci Sports 2015;25:880-7. [Crossref] [PubMed]
- Teichtahl AJ, Urquhart DM, Wang Y, Wluka AE, Wijethilake P, O'Sullivan R, Cicuttini FM. Fat infiltration of paraspinal muscles is associated with low back pain, disability, and structural abnormalities in community-based adults. Spine J 2015;15:1593-601. [Crossref] [PubMed]
- Takayama K, Kita T, Nakamura H, Kanematsu F, Yasunami T, Sakanaka H, Yamano Y. New Predictive Index for Lumbar Paraspinal Muscle Degeneration Associated With Aging. Spine (Phila Pa 1976) 2016;41:E84-90. [Crossref] [PubMed]
- Zhang Y, Zhou Z, Wu C, Zhao D, Wang C, Cheng X, Cai W, Wang L, Duanmu Y, Zhang C, Tian W. Population-Stratified Analysis of Bone Mineral Density Distribution in Cervical and Lumbar Vertebrae of Chinese from Quantitative Computed Tomography. Korean J Radiol 2016;17:581-9. [Crossref] [PubMed]
- Wang L, Cheng XG, Su YB, Brown K, Xu L, Li K, Zhang CX, Zhang Y, Duanmu YY, Wu XB, Wang MY. Sex-related variations in cortical and trabecular bone of the femoral neck in an elderly Chinese population. Osteoporos Int 2017;28:2391-9. [Crossref] [PubMed]
- Li K, Zhang Y, Wang L, Duanmu YY, Tian W, Chen H, Yin L, Bo J, Wang Y, Li W, He L, Zhao WH, Xu SQ, Zhao LF, Zhou J, Wang FZ, Liu Y, Zhu L, Chen YZ, Zhang XL, Hao XG, Shi ZW, Wang JY, Shao JM, Chen ZJ, Lei RS, Ning G, Zhao Q, Jiang YH, Zhi YH, Li BQ, Chen X, Xiang QY, Wang L, Ma YZ, Liu SW, Cheng XG. The protocol for the Prospective Urban Rural Epidemiology China Action on Spine and Hip status study. Quant Imaging Med Surg 2018;8:667-72. [Crossref] [PubMed]
- Huang Y, Luo J, Liu X, Wu Y, Yang Y, Li W, Lv W, Hu Y. Gamma-Glutamyltransferase and Risk of Acute Coronary Syndrome in Young Chinese Patients: A Case-Control Study. Dis Markers 2018;2018:2429160. [Crossref] [PubMed]
- Addison O, Marcus RL, Lastayo PC, Ryan AS. Intermuscular fat: a review of the consequences and causes. Int J Endocrinol 2014;2014:309570. [Crossref] [PubMed]
- Kalichman L, Klindukhov A, Li L, Linov L. Indices of Paraspinal Muscles Degeneration: Reliability and Association With Facet Joint Osteoarthritis: Feasibility Study. Clin Spine Surg 2016;29:465-70. [Crossref] [PubMed]
- Hebert JJ, Kjaer P, Fritz JM, Walker BF. The relationship of lumbar multifidus muscle morphology to previous, current, and future low back pain: a 9-year population-based prospective cohort study. Spine (Phila Pa 1976) 2014;39:1417-25. [Crossref] [PubMed]
- Lee SH, Park SW, Kim YB, Nam TK, Lee YS. The fatty degeneration of lumbar paraspinal muscles on computed tomography scan according to age and disc level. Spine J 2017;17:81-7. [Crossref] [PubMed]
- Katzman WB, Miller-Martinez D, Marshall LM, Lane NE, Kado DM. Kyphosis and paraspinal muscle composition in older men: a cross-sectional study for the Osteoporotic Fractures in Men (MrOS) research group. BMC Musculoskelet Disord 2014;15:19. [Crossref] [PubMed]
- Crawford RJ, Filli L, Elliott JM, Nanz D, Fischer MA, Marcon M, Ulbrich EJ. Age- and Level-Dependence of Fatty Infiltration in Lumbar Paravertebral Muscles of Healthy Volunteers. AJNR Am J Neuroradiol 2016;37:742-8. [Crossref] [PubMed]
- Valentin S, Licka T, Elliott J. Age and side-related morphometric MRI evaluation of trunk muscles in people without back pain. Man Ther 2015;20:90-5. [Crossref] [PubMed]
- Aubrey J, Esfandiari N, Baracos VE, Buteau FA, Frenette J, Putman CT, Mazurak VC. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol (Oxf) 2014;210:489-97. [Crossref] [PubMed]
- Lang T, Koyama A, Li C, Li J, Lu Y, Saeed I, Gazze E, Keyak J, Harris T, Cheng X. Pelvic body composition measurements by quantitative computed tomography: association with recent hip fracture. Bone 2008;42:798-805. [Crossref] [PubMed]
- Frank-Wilson AW, Chalhoub D, Figueiredo P, Jonsson PV, Siggeirsdottir K, Sigurdsson S, Eiriksdottir G, Guethnason V, Launer L, Harris TB. Study AG-R. Associations of Quadriceps Torque Properties with Muscle Size, Attenuation, and Intramuscular Adipose Tissue in Older Adults. J Gerontol A Biol Sci Med Sci 2018;73:931-8. [Crossref] [PubMed]
- Lang T, Streeper T, Cawthon P, Baldwin K, Taaffe DR, Harris TB. Sarcopenia: etiology, clinical consequences, intervention, and assessment. Osteoporos Int 2010;21:543-59. [Crossref] [PubMed]
- Francis P, Lyons M, Piasecki M, Mc Phee J, Hind K, Jakeman P. Measurement of muscle health in aging. Biogerontology 2017;18:901-11. [Crossref] [PubMed]
- Marcell TJ. Sarcopenia: causes, consequences, and preventions. J Gerontol A Biol Sci Med Sci 2003;58:M911-6. [Crossref] [PubMed]
- Curtis E, Litwic A, Cooper C, Dennison E. Determinants of Muscle and Bone Aging. J Cell Physiol 2015;230:2618-25. [Crossref] [PubMed]
- Tomlinson DJ, Erskine RM, Morse CI, Winwood K, Onambele-Pearson G. The impact of obesity on skeletal muscle strength and structure through adolescence to old age. Biogerontology 2016;17:467-83. [Crossref] [PubMed]