
Relationship between markers of disease activity and progression in skeletal muscle of GNE myopathy patients using quantitative nuclear magnetic resonance imaging and 31P nuclear magnetic resonance spectroscopy
Introduction
GNE myopathy (GNEM) is a rare, autosomal recessive, progressive neuromuscular disorder, caused by mutations in the UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase gene (abbreviated as GNE), which is a bifunctional enzyme of the sialic acid biosynthetic pathway (1). Sialic acids play an important role in maintaining membrane stability (2), and a decrease in sialic acid production will result in a reduced sialylation, which is the incorporation of sialic acid in glycoproteins and glycolipids, found in large numbers in cellular membranes (3). Reduced levels of sialic acid were observed in a mouse model expressing mutated GNE (4) that develop a pathology resembling a rimmed vacuolar myopathy (4) and named after the autophagic byproducts called ‘rimmed vacuoles’ (5). The exact mechanisms leading to these aggregates are still unknown. Although some degree of inflammation is a common feature in (sporadic) inclusion body myositis (3), it is usually not observed in GNEM. GNEM is characterized by an onset typically in the third decade of life, with initial distal lower limb muscle atrophy as a clinical presentation and a gradual spreading to more proximal muscles and upper limb (5).
Qualitative T1-weighted nuclear magnetic resonance imaging (NMRI) revealed fatty infiltrations in all leg (6), thigh (6,7), gluteus (6,8) and forearm (9) muscles. The disorder is known for the relative preservation of the quadriceps (QUAD), yet affected by fatty replacement in the later stages of the disease (3). Currently, quantitative NMRI is being used more and more in the evaluation of the disease of neuromuscular disorders (10) such as Duchenne muscular dystrophy (DMD) (11-13), Becker muscular dystrophy (BMD) (14), facioscapulohumeral muscular dystrophy (FSHD) (15), limb-girdle muscular dystrophy type 2I (16), late-onset Pompe disease (17) and inclusion body myositis (18). Most of these quantitative NMRI studies included a muscle-to-muscle-based determination of fat fraction (Fat%), often derived from Dixon-based water-fat separation approaches, and used as an outcome measure for disease progression. In a recent publication, we have reported significant changes in disease progression (i.e., change in Fat% and contractile cross-sectional area or cCSA) that were detected over the course of one year in GNEM patients using water-fat separation-based quantitative NMRI, whereas functional and strength tests could not demonstrate this progression (19).
Other NMR parameters such as muscle water T2 and phosphorus (31P) NMR spectroscopy (NMRS) indices have also been investigated in neuromuscular disorders, although to a lesser extent (11,12,14,17,20). These NMR biomarkers may, in fact, indicate abnormalities related to the underlying pathophysiological mechanisms, and could, therefore, be observed as markers of ‘disease activity’ (10). Specifically, in the case of T2, using qualitative T2-weighted NMR images, hyperintensities have been observed in the tibialis anterior and the medio-posterior part of the thigh in GNEM patients (6,9). This qualitative approach used in diagnostic imaging, not in the least in inflammatory myopathies (21-26), is based on visual evaluation of contrast differences between normal and abnormal muscle tissue. Besides the subjective nature of visual scoring of the T2-weighted approach, patients showing systemic inflammation across all investigated muscles might be erroneously categorized as false negatives (26). Quantitative muscle water T2, however, cancel out these aforementioned limitations of T2-weighted NMRI. Due to the sensitivity of water T2 to changes in muscle composition, it has been proposed as a quantitative outcome measure in a number of neuromuscular disorders, including late-onset Pompe disease (17), DMD (12,20,27) and inflammatory myopathies such as dermatomyositis (28) and inclusion body myositis (18,29). A study in healthy volunteers has also illustrated changes in water T2 values with ageing (30). Quantitative water T2 evaluations allow to mutually compare patients and/or to assess the changes within patients in longitudinal studies.
The purpose of the current work was to assess changes of water T2 and 31P NMRS indices in a one-year longitudinal study of GNEM patients. We investigated the predictive value of these ‘disease activity’ indices, by determining their relationship with parameters reflecting the disease progression (i.e., change in Fat% and cCSA), and, hence, to assess whether these indices prove to constitute surrogate endpoints in the longitudinal evaluation of GNEM.
Methods
Study population
The NMR protocol was performed at baseline and at a 1-year interval in 10 GNEM patients (mean age, 47±15 years; range, 25–73 years; 5 male, 3 non-ambulant), as previously described (19), and 29 age-matched healthy control subjects (mean age, 48±14 years; range, 18–70 years; 17 male). The patients were included from the French cohort of the Ultragenyx GNE-Myopathy Disease Monitoring Program (GNEM-DMP UX001-CL401), participating in the ClinBio GNE study. GNEM diagnosis was genetically confirmed. Healthy control subjects were scanned as part of a methodology NMRI/S protocol approved by the local ethics committee (CPP-Ile de France VI–Groupe Hospitalier Pitié-Salpêtrière, ID RCB: 2012-A01689-34) and informed consent was obtained from all controls and patients.
NMR acquisitions
NMR data were acquired on a 3-T clinical system (Trio until December 2014 or PrismaFit from January 2015, Siemens Healthineers, Erlangen, Germany). For quantitative NMRI, the system’s body coil for radiofrequency transmission was used with two 18-channel phase array surface coils (Siemens) covering both legs and thighs or a 4-phase array surface coil (Siemens) covering the dominant forearm, combined with a 32-channel spine coil (Siemens) for signal collection. Additionally, for 31P NMRS experiments, a dual-tuned 31P/1H transmit/receive surface coil (RAPID Biomedical GmbH, Rimpar, Germany) was placed over the thigh (centered at mid-femur), once on the anterior side, once on the posterior side, interrogating the QUAD and the hamstring (HSTR) muscles, respectively. We have described subject position in detail in a recently published work (19).
The NMR protocol began with a whole-body (3-point) Dixon acquisition (i.e., multiple 3D gradient echo sequences for quantitative water-fat imaging across the entire body), employing a methodology described in an earlier publication by some of the co-authors of this work (31). The objective of the whole-body Dixon measurement was to determine which of the segments demonstrated an overall Fat% >60%, which was determined as the upper limit for performing further quantitative NMRI and 31P NMRS.
Indeed, the actual quantitative NMRI protocol in this study comprised the acquisition of 3-point Dixon and water T2 mapping NMRI sequences at the level of the legs, the thighs and the domination forearm, separately. Details of the 3-point Dixon NMRI sequence were described in a separate publication in GNEM (19). For water T2 mapping, a multi-slice multi-echo (MSME) sequence was employed, covering 3 slices in the forearm and 9 slices in legs and thighs (10 mm slice thickness) and the following acquisition parameters: a train of 17 equidistant echoes (8.7 to 147.9 ms for the forearm; 9.5 to 161.5 ms for the legs/thighs); TR = 4,000 ms (forearm) or 3,000 ms (legs/thighs); nominal flip angles = 90° and 180°; field-of-view = 104×128 mm2 (forearm) or 224×448 mm2 (legs/thighs); spatial resolution = 1×1 mm2 (forearm) or 1.4×1.4 mm2 (legs/thighs); bandwidth = 445 Hz/pixel; slice gap = 30 mm; acquisition time = 5 min 18 s (forearm) or 3 min 45 s (legs/thighs). For calculating the transmit field (B1+) spatial distribution, the XFL sequence (32), which is a fast gradient echo image, was run covering the same volume as the MSME sequence, with the following parameters: TR = 2,000 ms (forearm) or 4,750 ms (legs/thighs); TE = 1.78 ms, flip angle = 8°.
31P NMRS data were obtained from a non-localized 500-µs hard pulse excitation with a TR of 4,000 ms, 64 averages, a bandwidth of 3,000 Hz, 2,048 data points, and an acquisition time of 4 min 16 s. For additional details about the 31P NMRS acquisitions, we refer to earlier publications (11,20).
NMR processing
All quantitative NMRI data were processed using in-house written code. Regions of interest (ROIs) were drawn manually, by the same operator, both on the out-of-phase Dixon and the MSME images, using a free software tool (www.itksnap.org). The ROIs were drawn on both left and right side, except for the forearm where only the dominant side was assessed. For the determination of Fat% and CSA, ROIs were drawn, respectively on the Dixon and the MSME images, in different muscle groups and therefore including intermuscular fat: the QUAD muscle group (comprising vastus lateralis, vastus medialis, vastus intermedius and rectus femoris muscles) and the HSTR muscle group (comprising biceps femoris, semimembranosus and semitendinosus muscles) of the thigh; the triceps surae (TRIC) muscle group (comprising soleus, gastrocnemius medialis and gastrocnemius lateralis muscles), the fibularis (FIB) muscle group and the extensor (EXT_LEG) muscle group (comprising tibialis anterior and extensor digitorum muscles) of the leg; and the flexor (FLEX) muscle group (comprising flexor carpi radialis, flexor carpi ulnaris, flexor digitorum profundus and flexor digitorum superficialis muscles) and the extensor (EXT_FOREARM) muscle group (comprising extensor pollicis longus, extensor carpi radialis, extensor carpi ulnaris, extensor digiti minimi and extensor digitorum muscles) of the forearm.
Using the Dixon images, Fat% values (expressed in percentage) were computed as the ratio between the fat signal and the sum of the water and fat signals. The Fat% value per muscle group was determined as a weighted average across five central slices (corresponding to the five central slices acquired with MSME, see further on in the text). The values for CSA were calculated in the same muscle groups on the same slices on the co-registered MSME images. The CSA value per muscle group was a mean value across these slices. The contractile CSA (cCSA), defined as the lean muscle CSA corresponding to the muscle volume fraction containing the contractile apparatus, was determined as follows:
|
| [1] |
Indices of disease progression were the Fat% change after 1 year (ΔFat%, in %), the muscle transformation rate (Rmuscle_transf, in year−1), the (absolute) change in cCSA (ΔcCSA, in mm2), and the relative change in cCSA (ΔcCSArel, in %); and were calculated as follows (with baseline and year-1 abbreviated as BL and Y1):
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
For assessment of water T2, ROIs delineated the interior of the muscle avoiding fasciae and blood vessels (33). ROIs for water T2 were drawn FIB, in the individual muscles of EXT_LEG, TRIC, QUAD and HSTR, as well as in the muscle groups EXT_FOREARM and FLEX (because of the difficulty to distinguish the different muscles in these groups). The MSME images were processed based on a tri-exponential fitting procedure (33), which is a model that takes into account both water and fat components in the muscle tissue. Water T2 values were determined as a mean value within the drawn muscle ROIs, averaged across the five centrally acquired slices (Figure 1). The acquisition of a B1 map enabled voxel sorting by eliminating those voxels having a B1+-value outside the prescribed boundaries (i.e., lower than 80% or higher than 120% of the nominal flip angle). The threshold for abnormal water T2 was set at two standard deviations above mean muscle water T2 in healthy volunteers, which is approximately 39 ms on the system used in this work (33). Water T2 values of individual muscles were pooled, weighted by the size of the ROI, to compare with all other measured NMR indices which were all determined on a muscle group level.
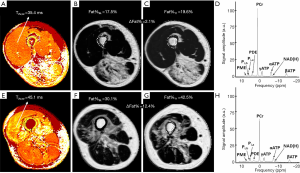
31P NMRS data were processed as previously described (11), using AMARES (34) in jMRUI (35) for the deconvolution of the two inorganic phosphate (Pi) resonances, and Topspin (Bruker Medical GMbG, Ettlingen, Germany) for the determination of PME (phosphomonoesters) and PDE (phosphodiesters). The following resonances were quantified: PME, Pi,b (alkaline inorganic phosphate), Pi,a (cytosolic inorganic phosphate), Pi,tot = Pi,b + Pi,a (total inorganic phosphate), PDE, PCr (phosphocreatine), γATP, αATP, NAD(H) (nicotonamide adenine dinucleotide) and βATP (adenosine triphosphate) (11). Prior knowledge was imposed on signal amplitude, line width, phases and line shape (11). All metabolites were expressed as metabolite ratios, corrected for partial saturation. The (weighted) pHw value was calculated based on the chemical shift difference between PCr and the Pi resonances (36). The intramuscular Mg2+ concentration was calculated based on the chemical shift difference between αATP and βATP (37,38). In Figure 1, two examples of 31P NMR spectra are given for patients with a different disease progression.
Statistical analysis
Statistical analyses were conducted using SPSS software version 22 (SPSS, Chicago, IL, USA). Given the small number of patients, non-parametric tests were performed, including the Mann-Whitney test for comparing control and patient values and the Wilcoxon test for comparing sides and 1-year differences. Ambulant and non-ambulant patients were pooled for all analyses. The Spearman-rank correlation test was used for investigating the relationship between variables reflecting ‘disease activity’ (water T2 and 31P NMRS) and the earlier assessed indices of disease progression (19). Water T2 values and 31P NMRS indices used for correlation analyses are presented as the average value between the baseline and year-1 visit. The level of statistical significance was corrected for multiple comparisons and set at P<0.007 and at P<0.02, for NMRI and 31P NMRS parameters, respectively.
The standardized response mean (SRM) is defined as the mean change over 1 year divided by the standard deviation of this change (39). An SRM ≥0.8 is considered to reflect a high responsiveness to change (39). For variables that are known to be less integrative but are, nevertheless, significantly different between the pathological and normal state, the standard SRM calculation is less pertinent. Therefore, we introduced an additional variable for the disease activity indices, which we further will call the ‘standardized difference mean’ (SDM). The SDM is defined as the sum of the mean change over 1 year and the mean difference between the patient and control group, divided by the pooled standard deviation of the 1-year change. Similar as for the SRM, an SDM ≥0.8 is considered to reflect a strong measurable difference from normality.
Results
Data overview
Quantitative NMRI data were obtained in the dominant forearm and the thigh of all ten GNEM patients. In four of the ten patients, no quantitative NMRI data were obtained in the leg due to overall Fat% values that were higher than 60%, as assessed by the whole-body Dixon measurement at the beginning of the NMR protocol. Three of these four patients were non-ambulant.
31P NMRS measurements were performed at the level of the QUAD in all ten GNEM patients. In four of the ten patients (including the three non-ambulant subjects), 31P NMRS was not obtained in the HSTR, due to very high Fat% values in HSTR muscles. A fourth patient felt discomfort and the NMR protocol was ended before the 31P NMRS measurement in the HSTR.
‘Disease progression’ NMR indices in patients
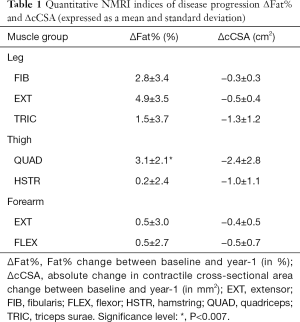
As can be observed from Table 1, changes in Fat% were significant for the QUAD. No other significant changes were observed.
Full table
Changes in ‘disease activity’ NMR indices between patients and controls
No significant age differences were found between patients and controls (P=0.292). There were no differences between left and right sides for water T2 values (P>0.05 for all muscles).
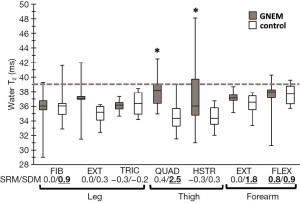
Compared to controls, significantly higher water T2 values (Figure 2) were only found in QUAD and HSTR muscles of GNEM patients, as depicted in Figure 2. SDM values for water T2 were high for QUAD, FIB and forearm muscles. No significant differences between muscles were observed for the average water T2 values in both patients and controls (P>0.05).
Additionally, anomalies in various 31P NMRS indices were observed in QUAD and HSTR as compared to controls (Figure 3). Corresponding SDM values for all depicted 31P NMRS indices were all higher than 0.8.
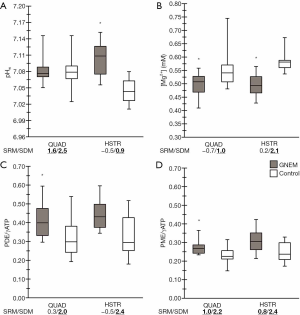
Significant differences between QUAD and HSTR were only found for pH in the healthy controls (7.08±0.03 vs. 7.05±0.02, P=0.003) and for PCr/γATP (5.56±0.39 vs. 4.89±0.33, P=0.016) in GNEM patients.
Changes in ‘disease activity’ NMR indices in patients after 1 year
Water T2 did not change after one year of follow-up. The SRM value in FLEX_FOREARM reached 0.8 but water T2 changes were non-significant (37.3±2.6 to 39.5±2.0 ms, P=0.022).
A few 31P NMRS indices changed significantly over the course of one year, such as increases in pHw (7.08±0.03 vs. 7.10±0.03, P=0.012, SRM =1.6) and PME/γATP (0.27±0.04 vs. 0.33±0.07, P=0.014, SRM =1.0) in QUAD. SRM reached 0.8 in HSTR for PME/γATP but changes were, however, non-significant after 1 year.
Correlations between ‘disease activity’ and ‘disease progression’ NMR indices
All correlation coefficients and corresponding P-values can be found in Table 2.
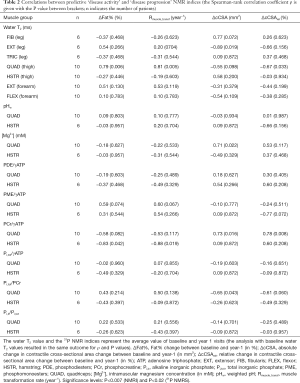
Full table
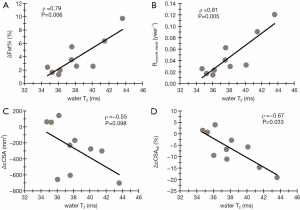
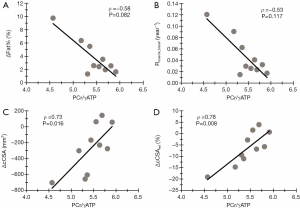
In QUAD, ΔFat% correlated significantly with water T2 (Figure 4). This significance was even more pronounced when correlating water T2 with the Rmuscle_transf index, as seen in Figure 4B. Water T2 values in QUAD seemed to be correlated (albeit non-significant) with the relative ΔcCSA values, as illustrated in Figure 4D. Additionally, significant correlations were found between PCr/γATP and ΔcCSA and ΔcCSArel in QUAD (Figure 5).
Discussion
This study demonstrates that water T2 and 31P NMRS have a predictive value on the progression of disease in skeletal muscles as established by fat-water separation-based quantitative NMRI in GNEM patients.
‘Disease progression’: ΔFat% and ΔcCSA
The extent of muscle destruction in GNEM, as reflected by the increased fatty infiltration and decreased lean muscle tissue as compared to controls, was especially evident in the thigh (QUAD and HSTR) and the extensor compartment of the leg, with a relative sparing of the QUAD muscle group as compared to the other muscles (19), confirming observations from earlier reports (3). Disease progression in GNEM, illustrated by both an increase in fatty depositions (ΔFat%) and a decrease in lean muscle tissue (ΔcCSA), was also seen in these three muscle groups (although only significant changes were seen for Fat% in QUAD). In general, all leg muscles in GNEM patients were heavily fatty infiltrated, confirming the distal-to-proximal disease evolution known in GNEM (40). Disease progression indices were handled in detail in a separate publication (19).
‘Disease activity’: water T2
An increase in water T2 might be related to different phenomena such as inflammation, myocytic lesions, cell necrosis or edema, and can therefore be regarded as a non-specific NMR biomarker reflecting ‘disease activity’ (10). Whereas the abovementioned variables, Fat% and cCSA, are more integrators of the global disease progression over time, water T2 reflects a more instantaneous phenomenon.
The significantly increased water T2, as found in this study in 25% of all QUAD and HSTR muscles, might be perceived as surprising, as it is known that inflammation is generally absent in GNEM (3). A similar apparent paradox has been observed in late-onset Pompe disease patients (17) where the increased water T2 could not be associated to an inflammatory cause, from which it is hypothesized that the abnormal water T2 values rather reflect myocytic lesions, possibly related to the rupture of lysosomes. In the patients assessed in our study, a significantly increased water T2 was only found in the thigh. This could be due to a lack of statistical power since leg data were not acquired in four of the ten patients due to very high Fat% levels (19). EXT_FOREARM muscles revealed to have an SDM similar as found in thigh, illustrating its potential as a muscle where water T2 might be significantly different between healthy and pathological muscle tissue. GNEM patients did not show any significant change in water T2 over a 1-year time period, which does not rule out fluctuations between these two time points. Altogether, these data indicate that the well-preserved QUAD is the most appropriate muscle group to assess disease evolution through water T2 measurements in GNEM.
‘Disease activity’: 31P NMRS
Although inter-patient differences in 31P NMRS indices were apparent, significant differences as compared to controls were found for only a few of these parameters. One of the prominent differences were the elevated levels of PDE, found in QUAD and HSTR, which reflects an increased turnover of phospholipids, indicating a metabolic disturbance at the level of the sarcolemmal membrane (41). Both in BMD and DMD, PDE was found to be an early marker during the disease, as changes were observed prior to any other NMR-detectable changes such as Fat% (12,14). When comparing our results to those of the BMD study where the patient age range (i.e., 20–60 years) was similar to the one in this work (i.e., 25–73 years), we can, however, not assess the possibility of PDE as an early-phase biomarker of the disease, since the relatively spared QUAD demonstrated already low to moderate fat replacement (i.e., between 4.3% and 29.7%) (19). Additionally, it is known that PDE correlates positively with age (42,43), making age a confounding factor for further interpretation. Also, an elevated pH value was found in the HSTR and QUAD (at least at year 1), when comparing to the controls. An alkaline pH has shown to be a biomarker for dystrophic muscle, as illustrated in several studies already, with a normal pH in non-fat infiltrated DMD patients (12) compared to an alkaline pH when muscle began to show fatty replacement (11,12,20). An elevated pH reflects an increase in the second more alkaline Pi pool (Pi,b), which is hypothesized to originate from suffering myocytes or from an expanded interstitial space, as was demonstrated in dystrophic muscle (11,20). Increased PDE, as a marker of membrane disturbance, could result from abnormal or insufficient glycosylation/sialylation of important transmembrane glycoproteins, such as the voltage-gated sodium channels or α-dystroglycan as part of the dystrophin-glycoprotein complex (44). Dysfunction of these proteins could result in a reduced mechanostability of the sarcolemma, causing leaky membranes and a disturbed ionic homeostasis with implications on the value of pH. Further studies are required to investigate the dynamics of pH and PDE before the occurrence of fatty depositions in the muscle of GNEM patients. Finally, a significantly decreased intramuscular Mg2+ content, as was observed in both QUAD and HSTR, is also an interesting finding, knowing that Mg2+ is an essential and one of the most effective metal ions with respect to the activity of N-acetylmannosamine kinase activity, being one of the two GNE-coded enzymes (45,46). Despite the difference in Fat% between QUAD and HSTR (4.3–29.7% and 8.1–86.4%, respectively) (19), only the PCr/γATP ratio was shown to differ significantly between the two muscle groups. This illustrates that the very different extent of fatty infiltration in these muscle groups (at least up until a certain threshold) seemed to have, on average, little effect on the outcome of these parameters, whereas this correlation has been observed earlier in FSHD (47) and DMD (11). Significant changes coupled to high SRM values were only found for pH, PME/γATP and PCr/γATP in QUAD. This doesn’t rule out, nevertheless, the use of Mg2+ and PDE/γATP as biomarkers that are potentially subjective to treatment effects, since they show, just as pH and PME/γATP, a high degree of discriminative power between patients and controls (SDM ≥0.8) in both QUAD and HSTR.
Relationship between ‘disease activity’ and ‘disease progression’
We demonstrated that QUAD muscles of GNEM patients with abnormal water T2 experienced a faster disease progression, as seen with most variables reflecting ‘disease progression’. A similar relationship was observed in late-onset Pompe disease patients (17) where an elevated water T2 implied a doubling of the fatty infiltration rate. Along the same line, convincing evidence have been repeatedly produced in FSHD, a disease where bursts of toxic gene expression and inflammation in a particular muscle result in its rapid destruction. Several studies have shown that muscle hyperintensities in STIR imaging or muscle water T2 increases in quantitative maps precede muscle fatty replacement in T1-weighted images or in fat fraction maps (48-50). Correlations between water T2 and disease progression indices were less clear in other lower limb and forearm flexor muscles in this study, most plausibly due to the higher degree of fatty replacement in these muscles (19), making a reliable estimation of water T2 more challenging (33). Also, fluctuations of water T2 during the course of the study might have impacted the correlation. Therefore, the average value of both visits was used for the correlation analysis since it reflects better the oscillating nature of the muscle water T2 during which the study was running. The PCr/γATP ratio, as a marker of metabolic efficiency of the (residual) muscle, was correlated with disease progression indices in the thigh muscles. In QUAD, a strong relationship between the macroscopic loss of muscle mass as reflected by a reduction in cCSA, and biochemical changes such as the decreased PCr/γATP ratio reflecting a loss of contractile tissue, was shown in that specific muscle that is relatively spared but where disease activity is, nevertheless, high. In DMD, a positive correlation was established between PCr/γATP and the grip strength in FLEX_FOREARM (51), which, in its turn, is strongly correlated with the amount of muscle tissue, as illustrated in GNEM (19). In HSTR, where fatty infiltration was, on average, much more pronounced, similar correlations were observed, more indirectly, between PCr/γATP and Rmuscle_transf, but not with cCSA changes. Finally, the tendency of correlation found between intramuscular Mg2+ and the loss in QUAD tissue suggests that Mg2+ could be a potential biomarker in future therapeutic interventions in GNEM.
A recent phase-3 trial demonstrated that the extended-release formulation of sialic acid did not improve muscle strength and function in GNEM patients, as compared to placebo-treated GNEM patients (52), although it should be mentioned that quantitative NMRI and 31P NMRS variables were not assessed in these patients. The study outcome might have been different, however, if these variables had been measured. While quantitative NMRI and several functional quantitative biomarkers have illustrated to be highly sensitive to disease progression, the regulatory agencies are still requesting evidence of a tangible clinical effect for a new treatment to be validated. There are many observations supporting the following chain of events in muscle disease: (I) muscle damage, (II) muscle destruction and fatty replacement, (III) strength loss, (IV) functional impairment, and finally (V) disability. The formal demonstration of this cascade is extremely challenging, though, because of the time-scale in most diseases. A normalization of water T2 or halting the muscle fatty replacement as measured with quantitative NMRI, reflecting an interruption of the succession of pathological mechanisms leading to disability, are both potential strong indicators of a successful therapeutic intervention.
Methodological aspects
The major methodological issue in this study was the combination of the limited amount of patients, which is due to the very low prevalence of the disease (estimated 1/1,000,000 worldwide) (3) and the heterogeneous phenotype inherent to the disease, emphasizing even more the need for objective outcome measures. Because the ambulant and non-ambulant cohorts were too small for a paired ambulant versus non-ambulant analysis, data were pooled. Despite the low number of patient data, however, we were able to detect significant changes and correlations in skeletal muscle over a 1-year period using quantitative NMRI and 31P NMRS. Nevertheless, more data is needed to confirm and verify correlations between ‘disease prediction’ and ‘disease progression’ NMR indices, since above a certain threshold of fatty infiltration (approximately 50%), water T2 assessment becomes unreliable (33) and 31P NMRS does not reach a sufficient signal-to-noise level due to lack of viable muscle tissue, as was, for example, the case for HSTR in this study.
Conclusions
To summarize, this study revealed that, in GNEM, water T2 and 31P NMRS are complementary NMR indices to the standard Fat% assessment for evaluating muscle changes during the course of the disease. More specifically, the QUAD, which is relatively preserved in GNEM, in patients with higher water T2 values showed a faster disease progression. The results obtained in this study confirm the ability of the use of water T2 and 31P NMRS as possible surrogate endpoints in longitudinal studies of neuromuscular disorders. Future steps could be the implementation of this quantitative NMR protocol in a larger patient cohort as well as in clinical trials before and after a suitable treatment.
Acknowledgments
The authors thank the patients and their families for participating in the study. We acknowledge Jean-Yves Hogrel (Neuromuscular Physiology Laboratory, Neuromuscular Investigation Center, Institute of Myology) as well as Melanie Annoussamy, Ferial Toumi, Melanie Villeret, Dominique Duchêne, Aurelie Chabanon and Gwenn Ollivier (I-Motion) for their contributions in this work.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/qims-20-39). AB reports personal fees from Ultragenyx pharmaceutical, during the conduct of the study; LS reports grants and personal fees from Avexis, grants and personal fees from Biogen, grants and personal fees from Roche, personal fees from Cytokinetics, personal fees from Sarepta, personal fees from Biophytis, personal fees from Pfizer, personal fees from Catabasis, personal fees from Lupin, grants and personal fees from Dynacure, personal fees from Audentes, outside the submitted work; PGC reports personal fees from Santhera, personal fees from Sanofi, personal fees from Sarepta, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: Healthy control subjects were scanned as part of a methodology NMRI/S protocol approved by the local ethics committee (CPP-Ile de France VI – Groupe Hospitalier Pitié-Salpêtrière, ID RCB: 2012-A01689-34) and informed consent was obtained from all controls and patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Eisenberg I, Avidan N, Potikha T, Hochner H, Chen M, Olender T, Barash M, Shemesh M, Sadeh M, Grabov-Nardini G, Shmilevich I, Friedmann A, Karpati G, Bradley WG, Baumbach L, Lancet D, Asher E, Ben , Beckmann JS, Argov Z, Mitrani-Rosenbaum S. The UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase gene is mutated in recessive hereditary inclusion body myopathy. Nat Genet 2001;29:83-7. [Crossref] [PubMed]
- Schauer R. Sialic acids: Fascinating sugars in higher animals and man. Zoology 2004;107:49-64. [Crossref] [PubMed]
- Nishino I, Carrillo-Carrasco N, Argov Z. GNE myopathy: current update and future therapy. J Neurol Neurosurg Psychiatry 2015;86:385-92. [Crossref] [PubMed]
- Malicdan MC V, Noguchi S, Nonaka I, Hayashi YK, Nishino I. A Gne knockout mouse expressing human GNE D176V mutation develops features similar to distal myopathy with rimmed vacuoles or hereditary inclusion body myopathy. Hum Mol Genet 2007;16:2669-82. [Crossref] [PubMed]
- Nonaka I, Noguchi S, Nishino I. Distal myopathy with rimmed vacuoles and hereditary inclusion body myopathy. Curr Neurol Neurosci Rep 2005;5:61-5. [Crossref] [PubMed]
- Tasca G, Ricci E, Monforte M, Laschena F, Ottaviani P, Rodolico C, Barca E, Silvestri G, Iannaccone E, Mirabella M, Broccolini A. Muscle imaging findings in GNE myopathy. J Neurol 2012;259:1358-65. [Crossref] [PubMed]
- Chu CC, Kuo HC, Yeh TH, Ro LS, Chen SR, Huang CC. Heterozygous mutations affecting the epimerase domain of the GNE gene causing distal myopathy with rimmed vacuoles in a Taiwanese family. Clin Neurol Neurosurg 2007;109:250-6. [Crossref] [PubMed]
- Chaouch A, Brennan KM, Hudson J, Longman C, McConville J, Morrison PJ, Farrugia ME, Petty R, Stewart W, Norwood F, Horvath R, Chinnery PF, Costigan D, Winer J, Polvikoski T, Healy E, Sarkozy A, Evangelista T, Pogoryelova O, Eagle M, Bushby K, Straub V, Lochmüller H. Two recurrent mutations are associated with GNE myopathy in the North of Britain. J Neurol Neurosurg Psychiatry 2014;85:1359-65. [Crossref] [PubMed]
- de Dios JKL, Shrader JA, Joe GO, McClean JC, Williams K, Evers R, Malicdan MCV, Ciccone C, Mankodi A, Huizing M, McKew JC, Bluemke DA, Gahl WA, Carrillo-Carrasco N. Atypical presentation of GNE myopathy with asymmetric hand weakness. Neuromuscul Disord 2014;24:1063-7. [Crossref] [PubMed]
- Carlier PG, Marty B, Scheidegger O, Loureiro de Sousa P, Baudin P-Y, Snezhko E, Vlodavets D. Skeletal muscle quantitative nuclear magnetic resonance imaging and spectroscopy as an outcome measure for clinical trials. J Neuromuscul Dis 2016;3:1-28. [Crossref] [PubMed]
- Wary C, Azzabou N, Giraudeau C, Le Louër J, Montus M, Voit T, Servais L, Carlier P. Quantitative NMRI and NMRS identify augmented disease progression after loss of ambulation in forearms of boys with Duchenne muscular dystrophy. NMR Biomed 2015;28:1150-62. [Crossref] [PubMed]
- Hooijmans MT, Niks EH, Burakiewicz J, Verschuuren JJGM, Webb AG, Kan HE. Elevated phosphodiester and T2 levels can be measured in the absence of fat infiltration in Duchenne muscular dystrophy patients. NMR Biomed 2017;30:e3667. [Crossref] [PubMed]
- Willcocks RJ, Rooney WD, Triplett WT, Forbes SC, Lott DJ, Senesac CR, Daniels MJ, Wang DJ, Harrington AT, Tennekoon GI, Russman BS, Finanger EL, Byrne BJ, Finkel RS, Walter GA, Sweeney HL, Vandenborne K. Multicenter prospective longitudinal study of magnetic resonance biomarkers in a large Duchenne muscular dystrophy cohort. Ann Neurol 2016;79:535-47. [Crossref] [PubMed]
- Wokke BH, Hooijmans MT, van den Bergen JC, Webb AG, Verschuuren JJ, Kan HE. Muscle MRS detects elevated PDE/ATP ratios prior to fatty infiltration in Becker muscular dystrophy. NMR Biomed 2014;27:1371-7. [Crossref] [PubMed]
- Kan HE, Scheenen TWJ, Wohlgemuth M, Klomp DWJ, van Loosbroek-Wagenmans I, Padberg GW, Heerschap A. Quantitative MR imaging of individual muscle involvement in facioscapulohumeral muscular dystrophy. Neuromuscul Disord 2009;19:357-62. [Crossref] [PubMed]
- Willis TA, Hollingsworth KG, Coombs A, Sveen ML, Andersen S, Stojkovic T, Eagle M, Mayhew A, de Sousa PL, Dewar L, Morrow JM, Sinclair CD, Thornton JS, Bushby K, Lochmüller H, Hanna MG, Hogrel JY, Carlier PG, Vissing J, Straub V. Quantitative muscle MRI as an assessment tool for monitoring disease progression in LGMD2I: a multicentre longitudinal study. PLoS One 2013;8:e70993. [Crossref] [PubMed]
- Carlier PG, Azzabou N, de Sousa PL, Hicks A, Boisserie JM, Amadon A, Carlier RY, Wary C, Orlikowski D, Laforêt P. Skeletal muscle quantitative nuclear magnetic resonance imaging follow-up of adult Pompe patients. J Inherit Metab Dis 2015;38:565-72. [Crossref] [PubMed]
- Morrow JM, Sinclair CDJ, Fischmann A, Machado PM, Reilly MM, Yousry TA, Thornton JS, Hanna MG. MRI biomarker assessment of neuromuscular disease progression: a prospective observational cohort study. Lancet Neurol 2016;15:65-77. [Crossref] [PubMed]
- Gidaro T, Reyngoudt H, Le Louër J, Behin A, Toumi F, Villeret M, Araujo ECA, Baudin PY, Marty B, Annoussamy M, Hogrel JY, Carlier PG, Servais L. Quantitative nuclear magnetic resonance imaging detects subclinical changes over 1 year in skeletal muscle of GNE myopathy. J Neurol 2020;267:228-38. [Crossref] [PubMed]
- Reyngoudt H, Turk S, Carlier PG. 1H NMRS of carnosine combined with 31P NMRS to better characterize skeletal muscle pH dysregulation in Duchenne muscular dystrophy. NMR Biomed 2018;31:e3839. [Crossref] [PubMed]
- Park JH, Vansant JP, Kumar NG, Gibbs SJ, Curvin MS, Price RR, Partain CL, James AE. Dermatomyositis: Correlative MR imaging and P-31 MR spectroscopy for quantitative characterization of inflammatory disease. Radiology 1990;177:473-9. [Crossref] [PubMed]
- Maillard SM, Jones Jones R, Owens C, Pilkington C, Woo P, Wedderburn LR, Murray KJ. Quantitative assessment of MRI T2 relaxation time of thigh muscles in juvenile dermatomyositis. Rheumatology (Oxford) 2004;43:603-8. [Crossref] [PubMed]
- Walker UA. Imaging tools for the clinical assessment of idiopathic inflammatory myositis. Curr Opin Rheumatol. 2008;20:656-61. [Crossref] [PubMed]
- Degardin A, Morillon D, Lacour A, Cotten A, Vermersch P, Stojkovic T. Morphologic imaging in muscular dystrophies and inflammatory myopathies. Skeletal Radiol 2010;39:1219-27. [Crossref] [PubMed]
- Yao L, Gai N. Fat-corrected T2 measurements as a marker of muscle disease in inflammatory myopathy. Am J of Roentgenol 2012;198:475-81. [Crossref]
- Carlier PG, Azzabou N, de Sousa PL, Florkin B, Deprez E, Romero NB, Denis S, Decostre V, Servais L. Diagnostic role of quantitative NMR imaging exemplified by 3 cases of juvenile dermatomyositis. Neuromuscul Disord 2013;23:814. [Crossref]
- Mankodi A, Azzabou N, Bulea T, Reyngoudt H, Shimellis H, Ren Y, Kim E, Fischbeck KH, Carlier PG. Skeletal muscle water T2 as a biomarker of disease status and exercise effects in patients with Duchenne muscular dystrophy. Neuromuscul Disord 2017;27:705-14. [Crossref] [PubMed]
- Marty B, Baudin PY, Reyngoudt H, Azzabou N, Araujo ECA, Carlier PG, de Sousa PL. Simultaneous muscle water T2 and fat fraction mapping using transverse relaxometry with stimulated echo compensation. NMR Biomed 2016;29:431-43. [Crossref] [PubMed]
- Bachasson D, Reyngoudt H, Turk S, Benveniste O, Hogrel JY, Carlier PG. Muscle alterations in sporadic inclusion body myositis assessed using quantitative nuclear magnetic resonance imaging and spectroscopy, ultrasound shear-wave elastography, and relationships with muscle function. Neuromuscul Disord 2017;27:S123. [Crossref]
- Azzabou N, Hogrel JY, Carlier PG. NMR based biomarkers to study age-related changes in the human quadriceps. Exp Gerontol 2015;70:54-60. [Crossref] [PubMed]
- Baudin PY, Marty B, Robert B, Shukelovitch A, Carlier RY, Azzabou N, Carlier PG. Qualitative and quantitative evaluation of skeletal muscle fatty degenerative changes using whole-body Dixon nuclear magnetic resonance imaging for an important reduction of the acquisition time. Neuromuscul Disord 2015;25:758-63. [Crossref] [PubMed]
- Amadon A, Cloos MA, Boulant N, Hang MF, Wiggins CJ, Fautz HP. Validation of a very fast B1-mapping sequence for parallel transmission on a human brain at 7T. Proc Intl Soc Mag Reson Med 2012;20:3358.
- Azzabou N, de Sousa PL, Araujo EC, Carlier PG. Validation of a generic approach to muscle water T2 determination at 3T in fat-infiltrated skeletal muscle. J Magn Reson Imaging 2015;41:645-53. [Crossref] [PubMed]
- Vanhamme L, van den Boogaart A, Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson 1997;129:35-43. [Crossref] [PubMed]
- Naressi A, Couturier C, Devos JM, Janssen M, Mangeat C, de Beer R, Graveron-Demilly D. Java-based graphical user interface for the MRUI quantitation package. MAGMA 2001;12:141-52. [Crossref] [PubMed]
- Moon RB, Richards JH. Determination of intracellular pH by 31P magnetic resonance. J Biol Chem 1973;248:7276-8. [PubMed]
- Wary C, Brillault-Salvat C, Bloch G, Leroy-Willig A, Roumenov D, Grognet JM, Leclerc JH, Carlier PG. Effect of chronic magnesium supplementation on magnesium distribution in healthy volunteers evaluated by 31P-NMRS and ion selective electrodes. Br J Clin Pharmacol 1999;48:655-62. [Crossref] [PubMed]
- Reyngoudt H, Lopez Kolkovsky AL, Carlier PG. Free intramuscular Mg2+ concentration calculated using both 31P and 1H NMRS-based pH in skeletal muscle of Duchenne muscular dystrophy patients. NMR Biomed 2019;32:e4115. [Crossref] [PubMed]
- Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale: L. Erlbaum Associates, 1988.
- Broccolini A, Gidaro T, Morosetti R, Mirabella M. Hereditary inclusion-body myopathy: Clues on pathogenesis and possible therapy. Muscle Nerve 2009;40:340-9. [Crossref] [PubMed]
- Srivastava NK, Yadav R, Mukherjee S, Pal L, Sinha N. Abnormal lipid metabolism in skeletal muscle tissue of patients with muscular dystrophy: In vitro, high-resolution NMR spectroscopy based observation in early phase of the disease. Magn Reson Imaging 2017;38:163-73. [Crossref] [PubMed]
- Younkin DP, Berman P, Sladky J, Chee C, Bank W, Chance B. 31P NMR studies in Duchenne muscular dystrophy: age-related metabolic changes. Neurology 1987;37:165-9. [Crossref] [PubMed]
- Reyngoudt H, Marty B, Baudin PY, Azzabou N, Carlier PG. Multinuclear NMR spectroscopic biomarkers for energy metabolism characterization in aging skeletal muscle. J Cachexia Sarcopenia Muscle 2015;6:487.
- Marini M, Ambrosini S, Sarchielli E, Thyrion GDZ, Bonaccini L, Vannelli GB, Sgambati E. Expression of sialic acids in human adult skeletal muscle tissue. Acta Histochem 2014;116:926-35. [Crossref] [PubMed]
- Hinderlich S, Sonnenschein A, Reutter W. Metal ion requirement of bifunctional UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase from rat liver. BioMetals 1998;11:253-8. [Crossref] [PubMed]
- Darvish D. Magnesium may help patients with recessive hereditary inclusion body myopathy, a pathological review. Med Hypotheses 2003;60:94-101. [Crossref] [PubMed]
- Kan HE, Klomp DWJ, Wohlgemuth M, van Loosbroek-Wagemans I, van Engelen BGM, Padberg GW, Heerschap A. Only fat infiltrated muscles in resting lower leg of FSHD patients show disturbed energy metabolism. NMR Biomed 2010;23:563-8. [Crossref] [PubMed]
- Friedman SD, Poliachik SL, Otto RK, Carter GT, Budech CB, Bird TD, Miller DG, Shaw DW. Longitudinal features of STIR bright signal in FSHD. Muscle Nerve 2014;49:257-60. [Crossref] [PubMed]
- Fatehi F, Salort-Campana E, Le Troter A, Lareau-Trudel E, Bydder M, Fouré A, Guye M, Bendahan D, Attarian S. Long-term follow-up of MRI changes in thigh muscles of patients with facioscapulohumeral dystrophy: A quantitative study. PLoS One 2017;12:e0183825. [Crossref] [PubMed]
- Monforte M, Laschena F, Ottaviani P, Bagnato MR, Pichiecchio A, Tasca G, Ricci E. Tracking muscle wasting and disease activity in facioscapulohumeral musculardystrophy by qualitative longitudinal imaging. J Cachexia Sarcopenia Muscle 2019;10:1258-65. [Crossref] [PubMed]
- Hogrel JY, Wary C, Moraux A, Azzabou N, Decostre V, Ollivier G, Canal A, Lilien C, Ledoux I, Annoussamy M, Reguiba N, Gidaro T, Le Moing AG, Cardas R, Voit T, Carlier PG, Servais L. Longitudinal functional and NMR assessment of upper limbs in Duchenne muscular dystrophy. Neurology 2016;86:1022-30. [Crossref] [PubMed]
- Lochmüller H, Behin A, Caraco Y, Lau H, Mirabella M, Tournev I, Tarnopolsky M, Pogoryelova O, Woods C, Lai A, Shah J, Koutsoukous T, Skrinar A, Mansbach H, Kakkis E, Mozaffar T. A phase 3 randomized study evaluating sialic acid extended-release for GNE myopathy. Neurology 2019;92:e2109-17. [PubMed]


