
Acute type A aortic intramural hematoma and type A aortic dissection: correlation between the intimal tear features and pathogenesis
Introduction
Aortic intramural hematoma (IMH) is a common acute aortic syndrome which can cause several adverse outcomes if no surgical or interventional treatment is given. Acute type A aortic IMH (ATAIMH), the same form with acute type A aortic dissection (AAAD), can be life-threatening and may result in disease progression along with the aorta (1).
Intimal tear is considered as a risk factor for worse prognosis in IMH patients. Ulcer-like projections (ULPs) is another hazard associated with IMH progression, and they are defined as structures filled with contrast media in the thickened aortic media (2). Studies suggested that ULPs may be caused by intimal micro-tears and occurred more commonly in the proximal aortic segment (3,4).
The relationship between intimal tear and IMH is still unclear. It was proposed that IMH is caused by spontaneous bleeding from the vasa vasorum into the aortic media, which is different from aortic dissection (AD) arising from intimal tear (5). However, multidetector computed tomography (MDCT) imaging showed the presence of small intimal tears in a variable percentage of patients between 71% and 78% diagnosed with IMH (6-8). Surgical studies also confirmed intimal disruption of 73% in the IMH patients, including those without evidence of the tear in preoperative MDCT as shown in 51.9% of patients (9,10).
Recently, with increased temporal and spatial resolution as well dramatic reduction in radiation and contrast medium dosage, MDCT has been increasingly used in the diagnosis, preoperative planning, and follow-up of patients with acute aortic syndromes. Electrocardiogram (ECG)-gated aortic computed tomography angiography (CTA) permits the correction of artefacts from arrhythmias or motions and allows the identification of small intimal disconnections in ATAIMH patients with a high accuracy (11). However, there has been no detailed analysis of the correlation between the intimal tear imaging features and the development of ATAIMH and AAAD. Thus, the purpose of this study was to clarify this relationship using ECG-gated CTA in both groups of patients based on a single center experience.
Methods
Patients
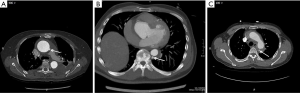
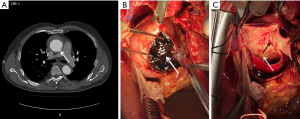
The study was approved by the local hospital ethics committee, and informed consent was obtained from all patients. Seventy-two consecutive ATAIMH and 209 consecutive AAAD patients diagnosed by ECG-gated MDCT were enrolled in this study between September 2015 and October 2016 (Figure 1). MDCT scan was performed within 2 weeks of the disease onset. IMH was defined as a crescentic or circular aortic wall thickening observed on CTA that was greater than 7 mm without a demonstrable intimal flap (12). No contrast enhancement was observed within the IMH on CTA images except for ulcer-like lesions (64/72, 88.9%), branch artery pseudoaneurysms (12/72, 16.7%) and intramural blood pools (5/72, 6.9%) (Figure 2) (13,14). Patients with iatrogenic AD as well as those with post-operative or endovascular aortic repair were excluded. Besides, those diagnosed with penetrating atherosclerotic ulcers (PAUs) containing localized IMH or adherent thrombus were also eliminated. These lesions were often accompanied by atheromatous plaques and intimal calcification.
Clinical management
All patients were admitted to the intensive care unit. Intravenous adrenergic receptor blocking agents (esmolol), calcium channel antagonists, and nitroprusside sodium either alone or in combination with nitroglycerin were administrated to maintain the systolic blood pressure within 100–120 mmHg and the heart rate lower than 70 beats per minute.
Emergent surgery was performed in the ATAIMH patients with complications such as aortic rupture, cardiac tamponade, progression to AAAD, or symptoms without resolution or even exacerbation after medical management. Surgery was delayed for those who were hemodynamically stable on admission. The AAAD patients received urgent or emergent surgery. The surgical findings were recorded for each patient.
CT data acquisition and image processing
All examinations were performed using a second-generation 128-slice dual-source CT (Somatom Definition Flash, Siemens Healthcare, Forchheim, Germany) with prospectively ECG-triggered high-pitch spiral acquisition or a 256-slice, 16 cm coverage CT system (Revolution CT, GE Healthcare, USA). All CT studies were executed in a craniocaudal direction starting from the thoracic inlet to the superior margin of the pubic symphysis.
A total of 70–85 mL of contrast medium (350 mgI/mL; Iopamidol, Iopamiron 300; Bayer Schering Pharma, Berlin, Germany) was administered with a dual-head power injector. The injection rate was adjusted according to the body mass index (BMI) and ranged from 3.0 to 4.5 mL/s. The density of the ascending aorta was monitored after the injection of the contrast medium. CT data acquisition was initiated when the density in the ascending aorta was greater than 200 Hounsfield units.
The axial image reconstruction parameters were as follows: 512×512 matrix; slice thickness and interval of 1 mm. All the images were analyzed using a separate workstation (Vitrea FX Workstation, Vital Images, Minnetonka, USA). The images were reviewed by two radiologists with over five years of experience in cardiac CT interpretation (Y.L. 18 years and N.Z. 9 years). Axial and multiplanar reformations (MPR) as well as volume-rendered and maximum intensity projection images of the aorta were produced.
Analysis of CT and clinical data
The maximum dimension of the intimal tear was measured. The location of each intimal tear, that is, within the ascending aorta, aortic arch, proximal descending aorta, distal descending aorta, proximal abdominal aorta or distal abdominal aorta (bound by the renal arteries), was documented. The intimal tear was defined as the continuity disruption of the inner layer, displayed as localized blood-filled pouch protruding into the thrombosed lumen of the aorta in the aortic axial and longitudinal interactive MPR images. In this study, the aortic axial images were obtained from MPR views perpendicular to the long axis of the aorta to avoid misidentification (Figure 3). Branch artery pseudoaneurysms were considered when a localized contrast pooling in the aortic wall was connected to the branch artery and the true lumen, and the small orifice of the true lumen was regarded as an intimal defect (Figure 2C) (15).
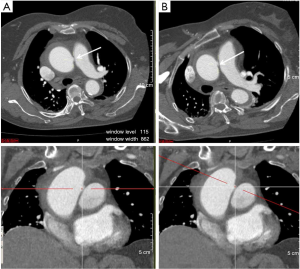
The extent of IMH and AD was described in terms of the involved segments (ascending aorta-1 segment, aortic arch-1, proximal descending aorta-1, distal descending aorta-1, proximal abdominal aorta-1 or distal abdominal aorta-1).
Statistical analysis
Statistical analysis was performed using SPSS software (version 11.5; SPSS, Chicago, IL, USA). The quantitative data were expressed as mean ± SD, and the categorical data were represented as proportions or percentages. Interobserver agreement with a 95% confidence interval (CI) was calculated using kappa statistics. Correlation between the mean scores for the overall motion artifact and the vessel lumen attenuation was estimated using Pearson product-moment correlation. A probability P value of <0.05 was considered statistically significant.
Results
Patient characteristics
Patients with IMH represented 18% with type A 6% and type B 12% of all cases of acute aortic syndromes. Patient demographics and disease characteristics are summarized in Table 1. Individuals with ATAIMH were older than those with AAAD (58.6±11.3 vs. 49.4±12.8 years, P<0.001), and females were predominant in the ATAIMH group (44.4% vs. 27.8%). Among the risk factors, Marfan syndrome was highly associated with the occurrence of AAAD. Pregnancy happened only in the AAAD patients. Besides, the ATAIMH patients presented with leg ischemia less frequently than the AAAD patients. However, no difference was noted in the other risk factors.
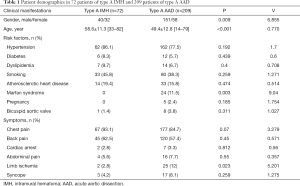
Full table
CTA findings
A total of 64 patients (88.9%) with IMH were recognized as having an intimal tear in the CTA images (Table 2). The average size of the tear was 5.54±2.06 mm and ranged from 1 to 15 mm, which was significantly smaller than that observed in the AAAD patients (25.93±13 mm). Among the first intimal tears (along the course of the aorta), 55 (85.9%) were located in the ascending aorta and aortic arch, which is similar to the distribution of tears in the AAAD patients (189/209) (90.4%, P=0.307). However, tears in the proximal ascending aorta were more common in patients with AAAD than in those with ATAIMH. When compared with AAAD, a higher number of ATAIMH patients presented with pleural (44% vs. 29.7%) and pericardial (43.1% vs. 16.7%) effusions; nonetheless, lesser coronary artery involvement was observed (4.2% vs. 38.3%; P<0.001). In the ATAIMH patients, 4.45±1.56 aortic segments were involved, which is significantly lower than that in the AAAD patients (5.04±1.72 segments, P<0.01). The interobserver agreement was excellent (k=0.823; 95% CI: 0.60–1.00).

Full table
Surgical findings
Surgical procedures
Twenty-eight ATAIMH patients received surgical management, including root-sparing ascending aortic replacement (n=1), root-sparing ascending aortic and partial arch replacement (n=3), root-sparing ascending aortic replacement and total arch replacement (n=19), Bentall + SUN’s procedure (n=4), and David and total arch replacement (n=1) (Videos 1 and 2). Coronary artery bypass grafting was simultaneously performed for two of the patients.
Pericardial hemorrhage
During the operation, pericardial hemorrhage was found in 17 patients (17/28, 60.7%), which was significantly higher than the observation made in AAAD patients (ATAIMH 60.7% (17/28) vs. AAAD 22.1% (27/122), P<0.001) and similar to those of the CTA findings (16/28, 57.1%).
Dissected aortic wall and intimal tear
In all the ATAIMH patients, fresh thrombus was seen to fill up the dissected aortic wall (Figure 4) during operation. During the surgical procedure, six ATAIMH patients were found to have progressed to classical AD, including an increase in the size of the intimal tear, and the dissected wall remained patent and filled with blood. All the intimal tears visualized on CTA were surgically confirmed (Table 3). Out of the 8 ATAIMH patients who did not demonstrate intimal tears on CTA, seven were confirmed to have the tears during operation. The size of the tear observed on CTA was smaller than that noted during surgery (CTA 6.96±5.12 mm vs. surgery 19.59±6.51 mm, P<0.001). The site of the dissection was similar in the two groups at the gross specimen (24 vs. 27 mm).
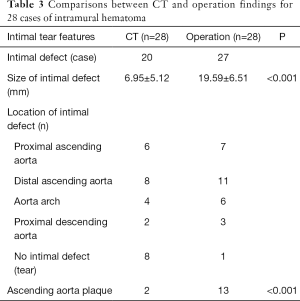
Full table
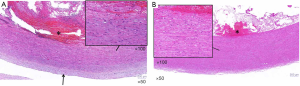
Lipid plaques were found at the site of the aortic intimal tear in nine patients during operation; however, this was not visible on the CTA images (Figure 4). Microscopically, intima thicken and fibrosis, the tear of the media and large number of blood cells gathered with extensive destruction of lamellar units (smooth muscle nucleus partially absent and degeneration of matrix) could be found in the IMH patients (Figure 5A), which may be different from the AD patients to some extent (Figure 5B).
Discussion
Unlike AD, the intimal layer always remains intact in IMH. Spontaneous bleeding from the vasa vasorum into the aortic media was considered to be the pathogenesis associated with IMH (5), but it was not confirmed by direct clinical or experimental evidence. Recently, MDCT images and surgical reports have shown the presence of small intimal tears in 58–78% of the IMH patients (6-8,10). Accordingly, the discussion whether “micro-intimal tear” is the true triggering event, and not vasa vasorum rupture, was prompted. The main findings of this study confirm that IMH is not a “hematoma in the aortic wall without intimal tear” but it rather originates from an intimal tear similar to classic AD. Nearly 90% of the patients diagnosed with IMH in this study were noted to have an intimal tear in the CT images. In some cases, the tears were not apparent in the preoperative CT images but were visualized during surgery. In other studies, about 50% of the patients did not have imaging evidence for the intimal tear on MDCT, but its presence was confirmed during the operation (9,10).
The higher prevalence of intimal tear in this study could be attributed to a number of reasons. First, CTA of the aorta was performed with a prospectively ECG-gated protocol, thereby avoiding the motion artifacts in the ascending aorta. Second, some of the micro-intimal tears were too small to be detected using the current imaging technology. In this research, axial CT images were reconstructed with a slice thickness and interval of 1 mm, which increased the spatial resolution and enhanced the detection of the micro-intimal tear (7). Third, MPR images were used to detect the small intimal tears. Axial images could miss such tears in the ascending aorta, especially in the tortuous ascending aortic path and the aortic arch.
Our hypothesis is that the dissections originate from an IMH; in other words, IMH occurs as a result of AD development. The intimal tear may be present at the site of the entry tear of AD. In this work, the intimal tears observed in CTA images were confirmed during the operation. Besides, the distribution of the intimal tear in ATAIMH was similar to that in the AAAD patients. Of the 72 patients with ATAIMH, four progressed to classic AAAD in the follow-up CT imaging. Six of the 28 ATAIMH patients who underwent surgery were diagnosed to have classic AD, and the intimal tear was the entry tear. The only case with intimal defect not detected during surgery is most likely due to the reason that the tear size is very small with thrombus formation around intimal tear, thus, leading to local healing which could not visualized or detected during operation. The size of the tear identified during operation was similar to that in the gross specimen and significantly larger than that in the CTA images. Similar findings were reported in other studies too (16,17). Dionne and Perrault reported that ATAIMH can progress rapidly to AAAD in two hours (18). Cho et al caught the split-second progression from ATAIMH to AAAD during the CT scan (19). It is nearly impossible to record the course of disease progression in every patient with AD. According to a recent histological analysis of IMH and AD, the initial event in both diseases develops in the outer third of the media (20). Uchida et al. demonstrated no significant differences in the severity of medial degeneration or necrosis between the IMH and AD groups (21). Thus, we presumed that IMH might result from an intimal tear and partially undergo a rapid progression to classic AD. In the population with Marfan syndrome and pregnancy, AAAD was more common than IMH. In these individuals, it is likely that the aortic media layer was more fragile than in other populations, such as those with hypertension.
Consistent with other studies (21,22), a higher incidence of mediastinal hematoma, pericardial effusion, and pleural effusion was noticed in patients with ATAIMH. All of the pericardial effusions in CT imaging were identified as pericardial hemorrhage during the surgery. The reason might be the higher pressure in the dissected aortic wall in the ATAIMH patients. There were at least two intimal tears in the AD, an entry tear from the lumen into the media and one or more reentry tear(s) back in the aortic lumen to release the pressure in the false lumen. On the other hand, there was only one entry tear in most of the IMH patients, and the pressure might have increased in the dissected aortic wall. This observation exhibits the positive correlation with adverse outcomes such as rupture and mortality in IMH (9,14,21).
Intimal tear in the ascending aorta frequently increases in size or progresses to classic dissection. Sandhu et al. also reported that the 30-day mortality in ATAIMH was not different from that noted in classic AAAD (23). A multidisciplinary management approach involving aggressive medical management and risk stratification for timely surgical intervention along with genetic profiling was recommended for optimal care. Long-term monitoring is mandatory to assess compliance with the medical therapy and to recognize the evolving complications.
Briefly, this study demonstrated that ATAIMH might be a subtype or precursor of AAAD even though some of the IMH patients showed complete resorption of the hematoma (22,24,25). To begin with, the risk factors, symptoms, and complications of ATAIMH were similar to those of AAAD and can result in life-threatening progressions such as rupture, hemothorax, tamponade, or conversion to AD (26-31). Both the conditions are classified by the same standards according to the involved extension. Moreover, the intimal tear was detected in more than 90% of the patients with ATAIMH and the distribution was similar to that observed in the AAAD patients, as reported in the literature (17,32). In this study, among all the operative cases, the site of the dissection was not different between the ATAIMH and AAAD patients in the surgical findings, while thrombus was more popular in ATAIMH. In the hypertension population, the lipid plaque in the intimal tear was discernable in both ATAIMH and AAAD, and there was no obvious difference in the incidence. In conclusion, just like classic AD, IMH might also be triggered by an intimal tear that results in a thrombosed false lumen owing to negligible re-entry into the true lumen.
Limitations
This study has several limitations. First, we focused on the first intimal tear along the aorta and considered it as the primary tear. The other tears distal to the left subclavian artery, branch artery pseudoaneurysms, and intramural blood pools were not analyzed. Their role in the progression should be followed-up in further investigations. Second, although we obtained the specimens of the intimal defect, the pathological findings were not compared between IMH and AD. Further, the range of the dissection was similar in the two groups, but it was not confirmed by the histopathological results. The residual media of the adventitial side was maintained to enclose the artificial vessel. Thus, the thickness of the adventitial side could not be measured in either of the groups. If IMH is triggered by spontaneous bleeding from the vasa vasorum into the media, the dissection may be significantly closer to the adventitia in the IMH group. Third, due to relatively younger age of participants in this study, reduction of radiation and contrast medium doses during CTA examinations is necessary with implementation of optimal scanning protocols as shown in other studies (33,34). Finally, follow-up of the ATAIMH and AAAD patients should be conducted in future studies.
In conclusion, this study shows that a high prevalence of intimal tear in patients with acute aortic IMH with the average size of the intimal tear measured significantly smaller than that in acute AD, and also with the involvement of fewer aortic segments. Results suggest that aortic IMH may be a stage of AD development, with the imaging studies portraying the clinical evolution of AD, which appears as fresh thrombus in the aorta during the operation.
Acknowledgments
The authors thank Zuo Huijuan for assistance in statistical design and analysis.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/qims-20-191). ZS serves as an unpaid associate editor of Quantitative Imaging in Medicine and Surgery. The other authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the local hospital ethics committee, and informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ahmad F, Cheshire N, Hamady M. Acute aortic syndrome: pathology and therapeutic strategies. Postgrad Med J 2006;82:305-12. [Crossref] [PubMed]
- Park GM, Ahn JM, Kim DH, Kang JW, Song JM, Kang DH, Lim TH, Song JK. Distal aortic intramural hematoma: clinical importance of focal contrast enhancement on CT images. Radiology 2011;259:100-8. [Crossref] [PubMed]
- Sueyoshi E, Matsuoka Y, Imada T, Okimoto T, Sakamoto I, Hayashi K. New development of an ulcerlike projection in aortic intramural hematoma: CT evaluation. Radiology 2002;224:536-41. [Crossref] [PubMed]
- Ganaha F, Miller DC, Sugimoto K, Do YS, Minamiguchi H, Saito H, Mitchell RS, Dake MD. Prognosis of aortic intramural hematoma with and without penetrating atherosclerotic ulcer: A clinical and radiological analysis. Circulation 2002;106:342-8. [Crossref] [PubMed]
- Macura KJ, Corl FM, Fishman EK, Bluemke DA. Pathogenesis in acute aortic syndromes: aortic dissection, intramural hematoma, and penetrating atherosclerotic aortic ulcer. AJR Am J Roentgenol 2003;181:309-16. [Crossref] [PubMed]
- Sawaki S, Hirate Y, Ashida S, Takanohashi A, Yagami K, Usui M. Clinical outcomes of medical treatment of acute type A intramural hematoma. Asian Cardiovasc Thorac Ann 2010;18:354-9. [Crossref] [PubMed]
- Kitai T, Kaji S, Yamamuro A, Tani T, Kinoshita M, Ehara N, Kobori A, Kim K, Kita T, Furukawa Y. Detection of intimal defect by 64-row multidetector computed tomography in patients with acute aortic intramural hematoma. Circulation 2011;124:S174-S178. [Crossref] [PubMed]
- Estrera AL, Sandhu HK, Leake SS, Charlton-Ouw KM, Afifi RO, Miller CC 3rd, Safi HJ. Early and late outcomes of acute type A aortic dissection with intramural hematoma. J Thorac Cardiovasc Surg 2015;149:137-42. [Crossref] [PubMed]
- Park KH, Lim C, Choi JH, Sung K, Kim K, Lee YT, Park PW. Prevalence of aortic intimal defect in surgically treated acute type A intramural hematoma. Ann Thorac Surg 2008;86:1494-500. [Crossref] [PubMed]
- Uchida K, Imoto K, Karube N, Minami T, Cho T, Goda M, Suzuki S, Masuda M. Intramural haematoma should be referred to as thrombosed-type aortic dissection. Eur J Cardiothorac Surg 2013;44:366-9. [Crossref] [PubMed]
- Valente T, Rossi G, Lassandro F, Rea G, Marino M, Muto M, Molino A, Scaglione M. MDCT evaluation of acute aortic syndrome (AAS). Br J Radiol 2016;89:20150825. [Crossref] [PubMed]
- Evangelista A, Mukherjee D, Mehta RH, O'Gara PT, Fattori R, Cooper JV, Smith DE, Oh JK, Hutchison S, Sechtem U, Isselbacher EM, Nienaber CA, Pape LA, Eagle KA. International Registry of Aortic Dissection (IRAD) Investigators. Acute intramural hematoma of the aorta: a mystery in evolution. Circulation 2005;111:1063-70. [Crossref] [PubMed]
- Williams DM, Cronin P, Dasika N, Upchurch GR Jr, Patel HJ, Deeb MG, Abrams G. Aortic branch artery pseudoaneurysms accompanying aortic dissection. Part I. Pseudoaneurysm anatomy. J Vasc Interv Radiol 2006;17:765-71. [Crossref] [PubMed]
- Wu MT, Wang YC, Huang YL, Chang RS, Li SC, Yang P, Wu TH, Chiou KR, Huang JS, Liang HL, Pan HB. Intramural blood pools accompanying aortic intramural hematoma: CT appearance and natural course. Radiology 2011;258:705-13. [Crossref] [PubMed]
- Williams DM, Cronin P, Dasika N, Kelly AM, Upchurch GR Jr, Patel HJ, Deeb MG, Nan B, Zheng J. Aortic branch artery pseudoaneurysms accompanying aortic dissection. Part II. Distinction from penetrating atherosclerotic ulcers. J Vasc Interv Radiol 2006;17:773-81. [Crossref] [PubMed]
- Disha K, Kuntze T, Girdauskas E. Unusual Case of Overt Aortic Dissection Mimicking Aortic Intramural Hematoma. Korean J Thorac Cardiovasc Surg 2016;49:126-9. [Crossref] [PubMed]
- Filippone G, Caruana G, Calia C, Moscaritolo V, Argano V. Evidence of intimal tear in type A intramural hematoma of the aorta: A case series. Int J Surg Case Rep 2018;42:179-81. [Crossref] [PubMed]
- Dionne PO, Perrault LP. Aortic intramural hematoma progressing rapidly to aortic dissection. Can J Cardiol 2014;30:1250.e23-5. [Crossref] [PubMed]
- Cho SH, Kim DH, Youn HC. Early progression of proximal intramural hematoma to overt aortic dissection during initial computed tomographic evaluation. J Formos Med Assoc 2017;116:324-5. [Crossref] [PubMed]
- Osada H, Kyogoku M, Ishidou M, Morishima M, Nakajima H. Aortic dissection in the outer third of the media: what is the role of the vasa vasorum in the triggering process? Eur J Cardiothorac Surg 2013;43:e82-e88. [Crossref] [PubMed]
- Uchida K, Imoto K, Takahashi M, Suzuki S, Isoda S, Sugiyama M, Kondo J, Takanashi Y. Pathologic characteristics and surgical indications of superacute type A intramural hematoma. Ann Thorac Surg 2005;79:1518-21. [Crossref] [PubMed]
- Song JK, Kim HS, Kang DH, Lim TH, Song MG, Park SW, Park SJ. Different clinical features of aortic intramural hematoma versus dissection involving the ascending aorta. J Am Coll Cardiol 2001;37:1604-10. [Crossref] [PubMed]
- Sandhu HK, Tanaka A, Charlton-Ouw KM, Afifi RO, Miller CC 3rd, Safi HJ, Estrera AL. Outcomes and management of type A intramural hematoma. Ann Cardiothorac Surg 2016;5:317-27. [Crossref] [PubMed]
- Song JK, Kang DH, Lim TH, Song MG, Kim JJ, Park SW, Park SJ. Different remodeling of descending thoracic aorta after acute event in aortic intramural hemorrhage versus aortic dissection. Am J Cardiol 1999;83:937-41. [Crossref] [PubMed]
- Kaji S, Akasaka T, Horibata Y, Nishigami K, Shono H, Katayama M, Yamamuro A, Morioka S, Morita I, Tanemoto K, Honda T, Yoshida K. Long-term prognosis of patients with type A aortic intramural hematoma. Circulation 2002;106:I248-52. [PubMed]
- Alomari IB, Hamirani YS, Madera G, Tabe C, Akhtar N, Raizada V. Aortic intramural hematoma and its complications. Circulation 2014;129:711-6. [Crossref] [PubMed]
- Maddu KK, Shuaib W, Telleria J, Johnson JO, Khosa F. Nontraumatic acute aortic emergencies: Part 1, Acute Aortic Syndrome. AJR Am J Roentgenol 2014;202:656-65. [Crossref] [PubMed]
- Gennari M, Arlati FG, Salvini L, Polvani G. Acute ascending aortic dissection associated with chronic intramural hematoma. J Card Surg 2016;31:628-30. [Crossref] [PubMed]
- Harris KM, Braverman AC, Eagle KA, Woznicki EM, Pyeritz RE, Myrmel T, Peterson MD, Voehringer M, Fattori R, Januzzi JL, Gilon D, Montgomery DG, Nienaber CA, Trimarchi S, Isselbacher EM, Evangelista A. Acute aortic intramural hematoma. An analysis from the International Registry of Acute Aortic Dissection. Circulation 2012;126:S91-6. [Crossref] [PubMed]
- Choi YJ, Son JW, Lee SH. Treatment patterns and their outcomes of acute aortic intramural hematoma in real world: multicenter registry for aortic intramural hematoma. BMC Cardiovasc Disord 2014;14:103. [Crossref] [PubMed]
- Zhou Y, Wang WC, Zhang XM, Yang C, Zheng J, Yang L, Dong L, Hu X, Zhu T, Wang YL, Yang Y. Aortic remodelling after thoracic endovascular aortic repair for acute and subacute type B aortic dissection. Quant Imaging Med Surg 2018;8:391-8. [Crossref] [PubMed]
- Filippone G, Caruana G, Moscaritolo V, Vacirca SR, Argano V. Radiologic and intraoperative finding of intimal tear in type A intramural hematoma of the aorta. Aorta (Stamford) 2018;6:92-3. [Crossref] [PubMed]
- Tan SK, Ng KH, Yeong CH, Raja Aman RRA, Mohamed Sani F, Abdul Aziz YF, Sun Z. Personalized administration of contrast medium with high delivery rate in low tube voltage coronary computed tomography angiography. Quant Imaging Med Surg 2019;9:552-64. [Crossref] [PubMed]
- Aldosari S, Jansen S, Sun Z. Optimization of computed tomography pulmonary angiography protocols using 3D printed model with simulation of pulmonary embolism. Quant Imaging Med Surg 2019;9:53-62. [Crossref] [PubMed]


