
CT-derived pulmonary vascular metrics and clinical outcome in COVID-19 patients
Introduction
Pathophysiological lung changes in patients affected by the novel Coronavirus Disease (COVID-19) are easily detectable with computed tomography (CT) and mirror the possible evolution from viral pneumonia to acute respiratory distress syndrome (ARDS) (1-7). Bilateral interstitial abnormalities are initially present as subpleural ground-glass opacities (GGOs), while progressive consolidations and reticular patterns can lately appear, reaching a large extent if cytokine dysregulation induces ARDS with diffuse alveolar and interstitial damage (1,3,5).
Extensive lung consolidation and ARDS can alter pulmonary vasculature features (8), engendering pulmonary hypertension (9,10), which in COVID-19 patients could also be caused by an overlap of these processes with increasingly-reported pulmonary arterial thrombosis (11-15). Early detection of pulmonary hypertension is therefore paramount to guide appropriate treatment (8) but remains a complex diagnostic challenge, also hampered by different and complementary shortcomings of available diagnostic techniques (10,16). While COVID-19 patients frequently undergo unenhanced CT, administration of iodinated contrast agents for CT pulmonary angiography, which would facilitate the diagnosis of pulmonary arterial thrombosis, may be contraindicated in COVID-19 patients. However, unenhanced CT measurements of the ratio between maximum diameters of the pulmonary artery (PA) and the ascending aorta (Ao)—PA/Ao ratio—were found to represent a helpful non-invasive option to detect pulmonary hypertension (9,10,16,17). A cut-off value of 29 mm for PA diameter is generally accepted as highly suggestive of pulmonary hypertension (16,18), while a PA/Ao ratio >0.9 has been found to be progressively correlated with pulmonary vascular impairment (9,10,17).
This retrospective study, performed in two hospitals near to the first area put in quarantine during the COVID-19 pandemic in Italy, aimed to evaluate intra-individual variations of pulmonary vascular metrics in COVID-19 patients, comparing unenhanced CT scans performed after emergency room (ER) admission and previous CTs performed for any reason except cardiovascular disease assessment, and to correlate vascular metrics to pneumonia extent (PnE) and clinical outcomes.
Methods
Study population
The local Ethics Committees approved this retrospective study (Ethics Committee of Brescia; protocol code NP 4154; approved on May 13, 2020).
COVID-19 patients who underwent thoracic CT at ASST Crema - Ospedale Maggiore, Crema, Italy; Center 1 and Fondazione Poliambulanza Istituto Ospedaliero, Brescia, Italy; Center 2 after ER admission from February 21 to March 21, 2020, were identified. At both institutions, during this timeframe, waiting times for reverse transcriptase-polymerase chain reaction (RT-PCR) results were too high to correctly triage the sheer number of suspected COVID-19 patients, CT being temporarily performed as a triaging test. We included in this study only patients who had subsequent confirmation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection by RT-PCR and had already performed another thoracic CT at the same institution for other clinical queries (except for cardiovascular disease): these previous CTs were considered as baseline examinations. Outcome data and arterial partial pressure of oxygen at admission (PaO2-Adm) were retrieved from clinical records.
Image acquisition
At Center 1, all examinations during the COVID-19 pandemic were performed on one of the two currently-installed 64×2-slices CT scanners (Aquilion CXL, Toshiba/Canon Medical Systems, Ōtawara, Japan, or Revolution EVO, General Electric Healthcare, Chalfont St. Giles, UK), while all baseline examinations had been performed on the formerly-installed 16-slices CT scanner (Diamond Select Brilliance 16, Philips Healthcare, Best, The Netherlands). At Center 2, all current and baseline examinations were performed on a 16-slices CT scanner (LightSpeed RT 16, General Electric Healthcare, Chalfont St. Giles, UK). Patients’ conditions were monitored during the procedure and all current scans were performed without the administration of iodinated contrast agents.
Image analysis
Two dedicated radiologists, with 7- and 20-year experience from each center, reviewed CTs from their own institution to assess pulmonary parenchyma, pulmonary vascular metrics, and Ao maximum diameter.
Lung parenchyma was assessed for the presence of the following features: GGOs and/or consolidation, crazy-paving pattern, pleural effusion, mediastinum lymphadenopathy. PnE was visually assessed according to the categories proposed by Bernheim et al. (2): 0% (absent); 1–25% (minimal); 26–50% (mild); 51–75% (moderate); over 75% (severe).
Perilesional vascular enlargement, defined as presence/absence of enlargement of pulmonary vessels next to lung parenchymal opacities, was qualitatively assessed (4).
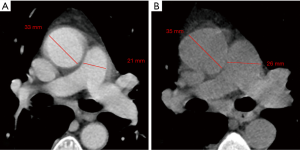
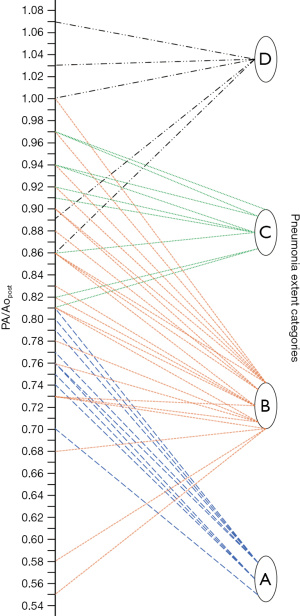
Finally, as described by Wells et al. (9), the PA maximum diameter at the level of its bifurcation and the Ao maximum diameter were assessed in a single slice (Figure 1), and PA/Ao ratio then calculated.
Statistical analysis
Data were reported as mean and standard deviation (SD) or as median and IQR according to their normal or non-normal distribution, assessed with the Shapiro-Wilk test. The Wilcoxon test was used to assess significant differences among PA/Ao ratio and PA maximum diameter before and after SARS-CoV-2 infection, Spearman’s ρ being used to assess correlations between vascular metrics, PnE, admission PaO2, and hospitalization length. The Mann-Whitney U test and the χ2 test were used to compare vascular metrics and PnE between groups with different outcomes.
Statistical analysis was performed using SPSS v.22.0 (IBM SPSS Inc., Chicago, IL, USA), and P values <0.05 were considered statistically significant.
Results
Population characteristics
In the study timeframe, 374 patients underwent triage chest CT for suspected SARS-CoV-2 infection at Center 1, 298 patients at Center 2. A previous CT examination was available for 45 of subsequently confirmed COVID-19 patients (median age 75.2 years, IQR 66.0–81.0 years), twenty-eight of them (62%) being males. At ER admission, 15 patients (33%) had both fever and dyspnea, 9 (20%) patients had both fever and cough, 13 (29%) only fever, 4 (9%) only dyspnea, 1 (2%) only cough, and 3 (7%) respiratory insufficiency. PaO2-Adm was available for 37 patients, with a median 70 mmHg (IQR 55–94 mmHg). Outcome data were available for 41/45 patients, 15/41 (37%) died after progression to severe ARDS and 26/41 (63%) discharged after a median 10 days hospitalization (IQR 0–20 days).
The previous CT scans used as baseline examinations were performed a median 36 months before ER admission (IQR 12–72 months).
Pulmonary parenchymal CT features
All but one patient had bilateral pneumonia, GGOs without consolidation being found in 29 (65%) patients, both GGOs and consolidation in 15 (33%), consolidations alone in one patient only (2%). Only one patient had associated crazy-paving. PnE was categorized as minimal in nine patients (20%), mild in 22 (49%), moderate in 9 (20%), and severe in 5 (11%). Pulmonary perilesional vascular enlargement was found in 10 patients (22%), 6 of them with bilateral consolidations. Five patients had bilateral pleural effusion and three others showed mediastinum lymphadenopathies.
Vascular metrics
Median PApre diameter was 26 mm (IQR 25–29 mm), median PApost diameter was 31 mm (IQR 28–33 mm) (P<0.001, Figures 1,2), and median overall ΔPApost-pre was 3 mm (IQR 1–5 mm). Median Aopre diameter was 35 mm (33–39 mm), while median Aopost diameter was 36 mm (IQR 33–39 mm) (P=0.005).
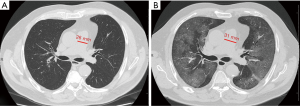
Median PA/Ao ratio at ER admission (0.83, IQR 0.76–0.92) was significantly higher (P<0.001) than at baseline (0.76, IQR 0.72–0.82), the median overall value of ΔPA/Aopost-pre being 0.07 (IQR 0.02–0.11).
Correlation between clinical data, radiological data, and outcome
In patients with unfavorable outcome (i.e., death), we found lower PaO2-Adm values (U=77, P=0.023) and worse PnE (P=0.024) than in discharged patients. Among vascular metrics, only the PApost diameter was significantly higher (U=74, P=0.001) in patients with unfavorable outcome.
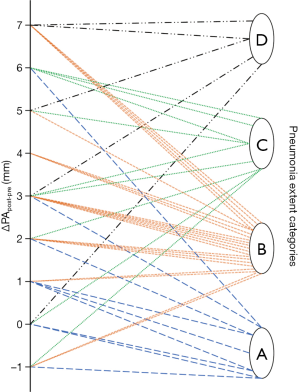
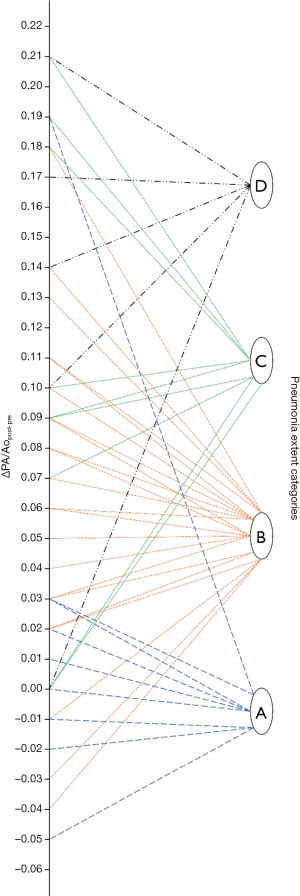
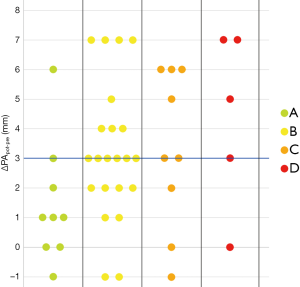
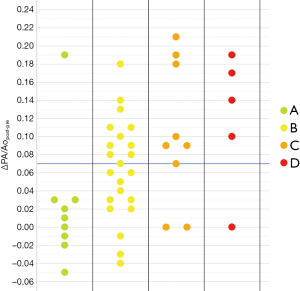
As noticeable in Figure 3, ΔPApost-pre values showed a significant but weak correlation with PnE (ρ=0.321, P=0.032). ΔPA/Aopost-pre values (Figure 4) showed a higher correlation with PnE (ρ=0.453, P=0.002), and a relatively higher correlation was also found between PA/Ao ratio at ER admission and PnE (ρ=0.631, P<0.001) (Figure 5). PaO2-Adm values likewise showed a significant but intermediate correlation with PnE (ρ=–0.459, P=0.004) and a significant but weak correlation with PA/Ao ratio at ER admission (ρ=–0.356, P=0.030). No other correlation was found among vascular metrics and the duration of hospitalization before discharge or death.
Discussion
Known disruption of pulmonary hemodynamics following large scale consolidation with inflammatory and vascular changes, expressed as vascular enlargement (4), could also be a concern in COVID-19 patients, which already present a frail intravascular equilibrium with high thromboembolic risk (15,19-25). Diagnosis of pulmonary hypertension is fraught with complications (8-10,16,17), but CT measurements of PA diameter and PA/Ao ratio constitute a helpful non-invasive option for the detection of pulmonary hypertension even in unenhanced CT, as demonstrated on large populations (9,17). While such metrics are easily attainable in COVID-19 patients which already perform unenhanced CT scans and could represent a potential prognostic index, to date no study to evaluate possible pulmonary hypertension has been conducted on these patients. We therefore performed this retrospective study considering two of the first clusters of patients in the Italian COVID-19 epidemic, aiming to compare intra-patient vascular metrics on chest CT performed before and during SARS-CoV-2 infection and their correlation with baseline PaO2 and patient outcome. Including only patients with a previous CT, our data comes from fragile patients, with a median age of 75 years and main reason to perform previous examinations of oncological nature.
When measuring PA diameter after SARS-CoV-2 infection we found a median value of 31 mm (IQR 28–33 mm), above the 27 mm and 29 mm reference values established for women and men, respectively, in the Framingham Heart Study (18). The large majority (37/45, 82%) of COVID-19 patients from our cohort presented an increased PA caliper compared to their baseline measurements. We also found an increase both in the median PA/Ao ratio and in the PA diameter absolute median value, that rose from 26 to 31 mm after SARS-CoV-2 infection. Of note, all ten patients without an increased PA/Ao ratio showed already high baseline values for both components of this parameter, suggesting that an increase in pulmonary vascular pression may be related to the inflammatory status triggered by SARS-CoV-2 infection.
While an intermediate correlation was found between PaO2-Adm and PnE, we found a weak correlation both between PaO2-Adm and PA/Aopost and between vascular metrics and PnE, as depicted in Figures 6,7. Considering the two categories with lowest PnE, we observed 9 patients with ΔPApost-pre values higher than the overall median value (Figure 6), and 11 patients with ΔPA/Aopost-pre higher than the overall median value (Figure 7). Conversely, considering the two categories with highest PnE, we observed 4 patients with ΔPApost-pre values lower than the overall median value and 3 patients with ΔPA/Aopost-pre lower than the overall median ΔPA/Aopost-pre. This could be explained considering that only one of our patients had already progressed to the late phase of mostly consolidative pulmonary damage, during which—as already demonstrated in various types of acute and chronic pulmonary interstitial disease (8-10,16,17)—pulmonary hypertension severity reaches its peak. However, a relatively better correlation with PnE was found for ΔPA/Aopost-pre than for ΔPApost-pre. Indeed, ΔPA/Aopost-pre is known to be a more robust parameter for the evaluation of pulmonary hypertension (10,17).
The overall weakness of these correlations, and especially the fact that only the PApost diameter was higher in patients who subsequently died than in discharged patients, suggest that pulmonary vascular metrics of COVID-19 patients and their relation with outcome are also influenced by factors other than PnE. Hypotheses to explain such findings could involve both a viral-induced autoimmune endothelial damage, already associated to other coronaviruses such as SARS-CoV-1 in the early 2000s (26), and a large spectrum of procoagulant abnormalities, increasingly observed in COVID-19 patients. These abnormalities include high levels of D-dimer and fibrin degradation products, presence of antiphospholipid antibodies, longer prothrombin time and activated partial thromboplastin time (15,19-25), resulting in pulmonary arterial thrombosis (13,14). Patients with a low increase in pulmonary metrics could be less affected by endothelial damage and thrombosis, already reported in COVID-19 patients with poor prognosis (12,19-25,27,28).
The observation that—among vascular metrics—only PA diameter was significantly higher in patients with unfavorable outcome than in discharged patients, could be related to a precocious manifestation of pulmonary arterial thrombosis. Thus, pulmonary vascular metrics on unenhanced CT scans could be useful to understand patients’ conditions, possibly working as a gate-keeper to contrast-enhanced CT, especially in patients with a mismatch between PnE and oximeter saturation data. Early detection of pulmonary hypertension at admission could also guide treatment of COVID-19 patients, triggering modifications of anticoagulation therapies from prophylactic to therapeutic dosage (21,27,29).
This study has limitations, the main one being the limited number of patients. We included all confirmed COVID-19 patients with a previous chest CT, performed for any reason (except cardiovascular diseases) at any time, even those with old previous CTs: this could bias the value of comparing previous pulmonary vascular metrics with those during infection. Moreover, tachypnoea and dyspnea are common in COVID-19 patients and motion artifacts during CT examinations could have influenced the measurements. Another limitation of our study is the manual measurement of great vessels diameters on axial scans, which is only a rough approximation for real vessels’ size. A more accurate approach would be to measure vessel areas on reformatted oblique reconstructions considering vessels’ axes, also considering the potential role of automatic recognition and measurement, already demonstrated for Ao on MRI (30). In addition, three-dimensional vascular metrics and flow parameters obtained by MRI or contrast-enhanced CT could also be considered in future studies.
In conclusion, COVID-19 patients showed higher median PA maximum diameter and median PA/Ao ratio compared to values measured on previous chest CTs performed for any reason except cardiovascular diseases. In this limited cluster of fragile patients, PA maximum diameter showed significant difference between patients with favorable and unfavorable outcome, a finding deserving further investigation as a potential driver of therapy decision-making.
Acknowledgments
Funding: This study was partially supported by Ricerca Corrente funding from Italian Ministry of Health to IRCCS Policlinico San Donato.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/qims-20-546). SS declares to be member of speakers’ bureau for General Electric and to have received travel support from Bracco. FS declares to have received grants from or to be member of speakers’ bureau/advisory board for Bayer, Bracco and General Electric. The other authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A. Coronavirus Disease 2019 (COVID-19): A Systematic Review of Imaging Findings in 919 Patients. AJR Am J Roentgenol 2020. Epub ahead of print. [Crossref] [PubMed]
- Bernheim A, Mei X, Huang M, Yang Y, Fayad ZA, Zhang N, Diao K, Lin B, Zhu X, Li K, Li S, Shan H, Jacobi A, Chung M, Chest CT. Findings in Coronavirus Disease-19 (COVID-19): Relationship to Duration of Infection. Radiology 2020;295:200463. [Crossref] [PubMed]
- Raptis CA, Hammer MM, Short RG, Shah A, Bhalla S, Bierhals AJ, Filev PD, Hope MD, Jeudy J, Kligerman SJ, Henry TS. Chest CT and Coronavirus Disease (COVID-19): A Critical Review of the Literature to Date. AJR Am J Roentgenol 2020. Epub ahead of print. [Crossref] [PubMed]
- Zhao W, Zhong Z, Xie X, Yu Q, Liu J. Relation Between Chest CT Findings and Clinical Conditions of Coronavirus Disease (COVID-19) Pneumonia: A Multicenter Study. AJR Am J Roentgenol 2020. Epub ahead of print. [Crossref] [PubMed]
- Xu R, Du M, Li L, Zhen Z, Wang H, Hu X. CT imaging of one extended family cluster of corona virus disease 2019 (COVID-19) including adolescent patients and “silent infection.” Quant Imaging Med Surg 2020;10:800-4. [Crossref] [PubMed]
- Zhang B, Zhang J, Chen H, Yang K, Zhang S. Unmatched clinical presentation and chest CT manifestation in a patient with severe coronavirus disease 2019 (COVID-19). Quant Imaging Med Surg 2020;10:871-3. [Crossref] [PubMed]
- Wang YXJ, Liu WH, Yang M, Chen W. The role of CT for Covid-19 patient’s management remains poorly defined. Ann Transl Med 2020;8:145. [Crossref] [PubMed]
- Beiderlinden M, Kuehl H, Boes T, Peters J. Prevalence of pulmonary hypertension associated with severe acute respiratory distress syndrome: Predictive value of computed tomography. Intensive Care Med 2006;32:852-7. [Crossref] [PubMed]
- Wells JM, Washko GR, Han MK, Abbas N, Nath H, Mamary AJ, Regan E, Bailey WC, Martinez FJ, Westfall E, Beaty TH, Curran-Everett D, Curtis JL, Hokanson JE, Lynch DA, Make BJ, Crapo JD, Silverman EK, Bowler RP, Dransfield MT. Pulmonary Arterial Enlargement and Acute Exacerbations of COPD. N Engl J Med 2012;367:913-21. [Crossref] [PubMed]
- Matsushita S, Matsuoka S, Yamashiro T, Fujikawa A, Yagihashi K, Kurihara Y, Nakajima Y. Pulmonary arterial enlargement in patients with acute exacerbation of interstitial pneumonia. Clin Imaging 2014;38:454-7. [Crossref] [PubMed]
- Marongiu F, Grandone E, Barcellona D. Pulmonary thrombosis in 2019-nCoV pneumonia? J Thromb Haemost 2020. Epub ahead of print. [Crossref] [PubMed]
- Dolhnikoff M, Duarte-Neto AN, de Almeida Monteiro RA, Ferraz da Silva LF, Pierre de Oliveira E, Nascimento Saldiva PH, Mauad T, Marcia Negri E. Pathological evidence of pulmonary thrombotic phenomena in severe COVID-19. J Thromb Haemost 2020. Epub ahead of print. [Crossref] [PubMed]
- Leonard-Lorant I, Delabranche X, Severac F, Helms J, Pauzet C, Collange O, Schneider F, Labani A, Bilbault P, Moliere S, Leyendecker P, Roy C, Ohana M. Acute Pulmonary Embolism in COVID-19 Patients on CT Angiography and Relationship to D-Dimer Levels. Radiology 2020. Epub ahead of print. [Crossref] [PubMed]
- Grillet F, Behr J, Calame P, Aubry S, Delabrousse E. Acute Pulmonary Embolism Associated with COVID-19 Pneumonia Detected by Pulmonary CT Angiography. Radiology 2020. Epub ahead of print. [Crossref] [PubMed]
- Xiong M, Liang X, Wei Y. Changes in Blood Coagulation in Patients with Severe Coronavirus Disease 2019 (COVID-19): a Meta-Analysis. Br J Haematol 2020. Epub ahead of print. [Crossref] [PubMed]
- Lewis G, Hoey ETD, Reynolds JH, Ganeshan A, Ment J. Multi-detector CT assessment in pulmonary hypertension: techniques, systematic approach to interpretation and key findings. Quant Imaging Med Surg 2015;5:423-32. [PubMed]
- Shen Y, Wan C, Tian P, Wu Y, Li X, Yang T, An J, Wang T, Chen L, Wen F. CT-Base Pulmonary Artery Measurement in the Detection of Pulmonary Hypertension. Medicine (Baltimore) 2014;93:e256. [Crossref] [PubMed]
- Truong QA, Massaro JM, Rogers IS, Mahabadi AA, Kriegel MF, Fox CS, O’Donnell CJ, Hoffmann U. Reference Values for Normal Pulmonary Artery Dimensions by Noncontrast Cardiac Computed Tomography. Circ Cardiovasc Imaging 2012;5:147-54. [Crossref] [PubMed]
- Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, Chen H, Ding X, Zhao H, Zhang H, Wang C, Zhao J, Sun X, Tian R, Wu W, Wu D, Ma J, Chen Y, Zhang D, Xie J, Yan X, Zhou X, Liu Z, Wang J, Du B, Qin Y, Gao P, Qin X, Xu Y, Zhang W, Li T, Zhang F, Zhao Y, Li Y, Zhang S. Coagulopathy and Antiphospholipid Antibodies in Patients with Covid-19. N Engl J Med 2020. Epub ahead of print. [Crossref] [PubMed]
- Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020;18:844-7. [Crossref] [PubMed]
- Ranucci M, Ballotta A, Di Dedda U, Bayshnikova E, Dei Poli M, Resta M, Falco M, Albano G, Menicanti L. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost 2020. Epub ahead of print. [Crossref] [PubMed]
- Han H, Yang L, Liu R, Liu F, Wu K, Li J, Liu X, Zhu C. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med 2020. Epub ahead of print. [Crossref] [PubMed]
- Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020. Epub ahead of print. [Crossref] [PubMed]
- Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V, Pesenti A, Peyvandi F, Tripodi A. Hypercoagulability of COVID-19 patients in Intensive Care Unit. A Report of Thromboelastography Findings and other Parameters of Hemostasis. J Thromb Haemost 2020. Epub ahead of print. [Crossref] [PubMed]
- Tan CW, Low JGH, Wong WH, Chua YY, Goh SL, Ng HJ. Critically Ill COVID-19 Infected Patients Exhibit Increased Clot Waveform Analysis Parameters Consistent with Hypercoagulability. Am J Hematol 2020. Epub ahead of print. [Crossref] [PubMed]
- Yang YH, Huang YH, Chuang YH, Peng CM, Wang LC, Lin YT, Chiang BL. Autoantibodies against human epithelial cells and endothelial cells after severe acute respiratory syndrome (SARS)-associated coronavirus infection. J Med Virol 2005;77:1-7. [Crossref] [PubMed]
- Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost 2020. Epub ahead of print. [Crossref] [PubMed]
- Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, Kucher N, Studt J-D, Sacco C, Alexia B, Sandri MT, Barco S. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res 2020. Epub ahead of print. [Crossref] [PubMed]
- Thachil J, Tang N, Gando S, Falanga A, Cattaneo M, Levi M, Clark C, Iba T. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost 2020. Epub ahead of print. [Crossref] [PubMed]
- Codari M, Scarabello M, Secchi F, Sforza C, Baselli G, Sardanelli F. Fully automated contour detection of the ascending aorta in cardiac 2D phase-contrast MRI. Magn Reson Imaging 2018;47:77-82. [Crossref] [PubMed]


