
New-onset non-convulsive status epilepticus in an adult with hemophagocytic lymphohistiocytosis: a case report
Introduction
Hemophagocytic lymphohistiocytosis (HLH) is a rare and life-threatening hyperinflammatory syndrome characterized by fever, hepatosplenomegaly, and cytopenia (1). In the past decades, an increasing amount of attention has been paid to the involvement of the central nervous system (CNS) in HLH. While neurological presentations are well described in HLH children, little is known about the involvement of CNS in HLH adults (2-4).
Status epilepticus (SE) is a clinical phenomenon characterized by prolonged or repetitive seizures for a certain period. Non-convulsive status epilepticus (NCSE) is a subtype of SE and is defined as prolonged or repetitive electrographic seizures lasting for more than 5 minutes without any motor manifestations (5). As clinical signs of this condition do not include overt symptoms or only include extremely nonspecific signs such as altered mental status, it is difficult to identify, which increases the risk for nondiagnosis (6). Herein, we report a rare case of HLH with CNS involvement manifested as NCSE in an adult woman.
Case report
A 52-year-old woman with a history of hypertension was admitted due to having a fever for 2 months. Initially, the local hospital’s laboratory examinations revealed leukocytosis (white blood cell count: 30.4×109/L; neutrophil ratio: 94.2%) and an elevated C-reactive protein level of 107.98 mg/L. Bacterial culture revealed a Staphylococcus infection. She was treated with antibiotics and antipyretics, but the symptoms did not improve. She developed pancytopenia, and the ferritin level increased (9,506 ng/mL). Positron emission tomography/computed tomography (PET/CT) showed splenomegaly and increased 18F-fludeoxyglucose uptake in multiple bones/medullae, and the bilateral cervical and axillary lymph nodes. The bone marrow biopsy performed by the local hospital showed hemophagocytosis. Lymph node biopsy and pathological examinations showed lymph node reactive hyperplasia with hemophagocytosis. HLH was suspected, and the patient was transferred to our hospital for further evaluation.
On admission, she was conscious, and routine examinations were otherwise unremarkable. Laboratory examinations showed pancytopenia, hyperferritinemia, and hypertriglyceridemia (Table 1). The pathological examination of the bone marrow performed at our hospital showed hemophagocytosis.
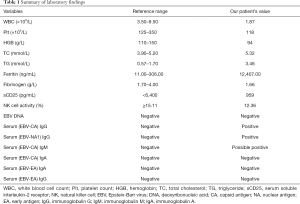
Full table
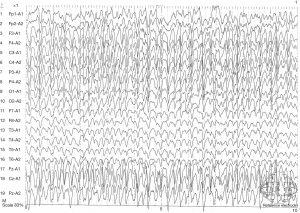
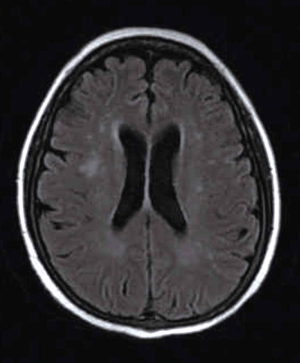
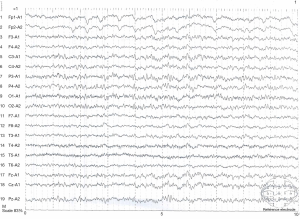
Based on these findings, secondary HLH was considered due to the lack of family history. Three days of chemotherapy was initiated with doxorubicin-etoposide-methylprednisolone (DEP) regimen (liposomal doxorubicin 40 mg on day 1, etoposide 150 mg on day 1, and methylprednisolone 80 mg on day 1, day 2, and day 3 and then decrease gradually). There were no significant adverse effects except for constipation after the first course of treatment. Then, the second course of chemotherapy with the same DEP regimen was done 1 month later. On the second day, the patient presented with alerted consciousness and abnormal behavior. She was unable to have breakfast, unable to go to the toilet, and unable to put on pants by herself. She could answer some easy questions, but her reaction was slow. There was no limb convulsion or urinary incontinence. Physical examinations showed reduced orientation, comprehension, memory, and calculation capabilities. There were no other abnormalities found in other neurological examinations. Electroencephalography (EEG) was performed, and results revealed continuous generalized rhythmic sharp-wave and slow-wave discharges (Figure 1), Magnetic resonance imaging (MRI) of the brain revealed patchy abnormal signals in the bilateral periventricular white matter in the fluid-attenuated inversion recovery (FLAIR) sequence (Figure 2). A lumbar puncture was also performed, and intracranial pressure was measured. The cerebrospinal fluid (CSF) was collected to measure protein content, glucose concentration, cell count, immunoglobulin synthesis rate, immunoglobulin G (IgG) index, and antibodies related to paraneoplastic and autoimmune encephalitis. The CSF-related parameters were in normal range, except for the mildly elevated immunoglobulin IgG (43.6 mg/dL; normal <34 mg/dL). Based on the clinical features and characteristic EEG findings, NCSE was diagnosed. As the therapeutic drugs currently used had no adverse effects on the seizures, we speculated that the current NCSE was related to HLH. After consulting with a neurologist and considering the patient’s general condition, oral levetiracetam 0.5 g twice daily was administered. The patient’s consciousness was improved on the second day after treatment; however, a second EEG revealed no improvements. On the third day, the patient’s consciousness dramatically improved. Physical examinations showed that the orientation, comprehension, calculation, and memory capabilities had returned to normal. The third EEG showed that the background significantly improved (Figure 3). The patient was still treated with oral levetiracetam at 0.5 g twice daily. She was followed up for more than 6 months, and no seizures or limb convulsions were observed.
Discussion
HLH is a hyperinflammatory syndrome with uncontrolled proliferation of the activated nonneoplastic lymphocytes and macrophages, resulting in phagocytosis of other blood cells and an overproduction of inflammatory cytokines. Clinically, HLH is categorized as a primary or familial HLH when the individual has a family history or a recognized genetic cause. Reactive or secondary HLH occurs with the onset of an infection, an underlying rheumatologic disorder, or a malignancy. Primary HLH is mainly found in children, and symptoms often occur before 2 years of age in 80% of patients. Secondary HLH can occur at any age. The clinical diagnosis of HLH is based on the presence of five out of eight HLH criteria in the absence of a gene mutation:
- Fever of >38.5 °C;
- Splenomegaly;
- Cytopenia of ≥2 cells lines (nadirs);
- Triglyceride ≥3.0 mmol/L and fibrinogen ≤1.5 g/L;
- Hemophagocytosis in the bone marrow, spleen, or lymph node;
- Low or absent nk cell activity;
- Ferritin ≥500 ng/mL;
- Soluble cd-25 ≥2,400 U/mL (7).
In our report, the patient presented with fever, pancytopenia, splenomegaly, hyperferritinemia, hypertriglyceridemia, decreased NK cell activity, and hemophagocytosis on bone marrow examination. Based on the family history and the lack of evidence for tumor or rheumatologic disorder, she was diagnosed with HLH secondary to infection.
The involvement of CNS was reported in different studies, occurring either at disease onset or during HLH (2,8,9). The most frequent neurological feature includes meningismus, disorder of consciousness, seizures, ataxia, palsy of cranial nerves, nystagmus, irritability, and intracranial hypertension. Reported neuroradiological findings in MRI include cerebral atrophy, parenchymal lesions with hypointensity or T2 hyperintensity, calcification, nodular or ring-enhancing parenchymal lesions, leptomeningeal and perivascular enhancements, and subdural fluid collection (8,10). The typical findings on a cytological examination of CSF are lymphocytic pleocytosis, activated monocytes, and hemophagocytosis. Unfortunately, current research on CNS involvement of HLH are from studies on HLH children; therefore, little is known about CNS involvement of HLH in adults (2,3). The latest single-center study of HLH adults showed that the incidence of CNS involvement was 20.7%. Among the patients with clinical symptoms, disturbance of consciousness was the most common symptom (39.6%), followed by headache/dizziness (24.0%), seizure (17.7%), and psychiatric symptoms (16.7%) (11). Although the disturbance of consciousness and seizure is considered as severe symptoms, the specific causes of a disturbance of consciousness and classification of seizures have not been further analyzed in the available studies.
NCSE is a particular type of seizure and can manifest with some different symptoms such as altered mental status, speech disturbance, myoclonus, unusual behaviors, anxiety, agitation, delirium, extrapyramidal signs, and hallucinations. Currently, NCSE is accepted as a continuous non-convulsive seizure that lasts >30 min or multiple non-convulsive seizures during a >30 min period in which sensory, motor, and/or cognitive function is not fully recovered (12). An EEG is the only tool for the definite diagnosis of NCSE as the clinical symptoms of NCSE are mild and sometimes even difficult to differentiate from normal behaviors (13). Currently, there are no evidence-based EEG criteria for NCSE. Typical EEG features for NCSE are repetitive generalized or focal spikes, poly spike, sharp waves, and spike-and-wave or sharp-and-slow wave complexes at >2.5/second in patients without a known epileptic encephalopathy (14). In addition, a good response to benzodiazepine in EEG and clinical symptoms can also be used as the basis for the diagnosis of NCSE when EEG changes are non-specific, although lack of immediate response to treatment does not necessarily exclude this diagnosis.
Our patient presented with alerted consciousness and behavioral abnormality. EEG showed continuous generalized sharp and slow-wave discharges. After an oral administration of levetiracetam, she became conscious, and the EEG background returned to normal. All her clinical manifestations and EEG characteristics met the diagnostic criteria for NCSE as summarized by Sutter and Kaplan (15). The etiology of NCSE is complex and may be different between children and adults. The following factors are related to the pathogenesis of NCSE:
- Metabolic or systemic diseases, or certain drugs such as antibiotics (cephalosporins, quinolones, and penicillin);
- Primary CNS dysfunction such as cerebral infarction, brain tumor, traumatic brain injury, encephalopathy, and ischemic hypoxia encephalopathy;
- For patients with epilepsy, inappropriate discontinuation of antiepileptic drugs, irregular use of antiepileptic drugs, or poor control of epilepsy.
In this case, there was no evidence of tumor, CNS infection, or metabolic disorder. The examination of antibodies related to the paraneoplastic antibodies and autoimmune encephalitis revealed negative results. There were no special antibiotics used in this patient. Therefore, we speculate that the NCSE in this HLH patient was secondary to HLH.
Treatment for NCSE should be initiated as quickly as possible. Intravenous benzodiazepines are recommended as the first-line therapy followed by phenytoin, phenobarbital, and then anesthetics (16). Oral antiepileptic drugs, instead of intravenous benzodiazepines, were used after considering our patient’s general condition. Fortunately, the NCSE was controlled 2 days later. We speculate that, although oral antiepileptic drugs are effective in the treatment of NCSE, intravenous treatment should be considered first in order to control seizures as quickly as possible.
No epidemiological studies have been conducted to examine the population-based incidence of NCSE. One study conducted in the surgical intensive care unit (ICU) showed that EEG could identify the NCSE-related EEG patterns in 16% of patients who had a disorder of consciousness but no brain abnormalities (17). Other studies in patients with altered mental status (AMS) reported that the prevalence of NCSE ranged from 16% to 37% (13,17,18).
As the clinical signs of NCSE are challenging to identify, the incidence of NCSE might be even higher, especially in patients with altered consciousness. To our knowledge, this was the first case of NCSE in HLH patients. Early recognition of NCSE allows for better control of seizures in our patient, although only oral levetiracetam was administered.
In summary, we, for the first time, report a case of new-onset NCSE in a woman with HLH. We speculate that incidence of NCSE might be underestimated in HLH patients. An EEG should be performed as soon as possible in HLH patients with altered consciousness, which is helpful for the early identification and subsequent treatment of NCSE.
Acknowledgments
We thank Doctor Lin Wu and Professor Zhao Wang (Department of Hematology, Beijing Friendship Hospital, Capital Medical University, Beijing, China) for sharing detailed information about this patient.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/qims-19-360). The authors have no conflicts of interest to declare.
Ethical Statement: Approval from the institutional review board and ethics committee was obtained before the study. Informed consent has been signed.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Janka GE. Familial and acquired hemophagocytic lymphohistiocytosis. Eur J Pediatr 2007;166:95-109. [Crossref] [PubMed]
- Cai G, Wang Y, Liu X, Han Y, Wang Z. Central nervous system involvement in adults with haemophagocytic lymphohistiocytosis: a single-center study. Ann Hematol 2017;96:1279-85. [Crossref] [PubMed]
- Gratton SM, Powell TR, Theeler BJ, Hawley JS, Amjad FS, Tornatore C. Neurological involvement and characterization in acquired hemophagocytic lymphohistiocytosis in adulthood. J Neurol Sci 2015;357:136-42. [Crossref] [PubMed]
- Horne A, Trottestam H, Arico M, Egeler RM, Filipovich AH, Gadner H, Imashuku S, Ladisch S, Webb D, Janka G, Henter JI. Frequency and spectrum of central nervous system involvement in 193 children with haemophagocytic lymphohistiocytosis. Br J Haematol 2008;140:327-35. [Crossref] [PubMed]
- Trinka E, Cock H, Hesdorffer D, Rossetti AO, Scheffer IE, Shinnar S, Shorvon S, Lowenstein DH. A definition and classification of status epilepticus--Report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia 2015;56:1515-23. [Crossref] [PubMed]
- Kubota Y, Nakamoto H, Kawamata T. Nonconvulsive Status Epilepticus in the Neurosurgical Setting. Neurol Med Chir (Tokyo) 2016;56:626-31. [Crossref] [PubMed]
- Henter JI, Horne A, Arico M, Egeler RM, Filipovich AH, Imashuku S, Ladisch S, McClain K, Webb D, Winiarski J, Janka G. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 2007;48:124-31. [Crossref] [PubMed]
- Jovanovic A, Kuzmanovic M, Kravljanac R, Micic D, Jovic M, Gazikalovic S, Pasic S. Central nervous system involvement in hemophagocytic lymphohistiocytosis: a single-center experience. Pediatr Neurol 2014;50:233-7. [Crossref] [PubMed]
- Yang S, Zhang L, Jia C, Ma H, Henter JI, Shen K. Frequency and development of CNS involvement in Chinese children with hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 2010;54:408-15. [Crossref] [PubMed]
- Rego I, Severino M, Micalizzi C, Faraci M, Pende D, Dufour C, Arico M, Rossi A. Neuroradiologic findings and follow-up with magnetic resonance imaging of the genetic forms of haemophagocytic lymphohistiocytosis with CNS involvement. Pediatr Blood Cancer 2012;58:810-4. [Crossref] [PubMed]
- Song Y, Pei RJ, Wang YN, Zhang J, Wang Z. Central Nervous System Involvement in Hemophagocytic Lymphohistiocytosis in Adults: A Retrospective Analysis of 96 Patients in a Single Center. Chin Med J (Engl) 2018;131:776-83. [Crossref] [PubMed]
- Sutter R, Semmlack S, Kaplan PW. Nonconvulsive status epilepticus in adults - insights into the invisible. Nat Rev Neurol 2016;12:281-93. [Crossref] [PubMed]
- Mesraoua B, Deleu D, Al Hail H, Ibrahim F, Melikyan G, Al Hussein H, Singh R, Uthman B, Streletz L, Kaplan PW, Wieser HG. Clinical presentation, epidemiology, neurophysiological findings, treatment and outcome of nonconvulsive status epilepticus: a 3-year prospective, hospital-based study. J Drug Assess 2017;6:18-32. [Crossref] [PubMed]
- Kaplan PW. EEG criteria for nonconvulsive status epilepticus. Epilepsia 2007;48 Suppl 8:39-41. [Crossref] [PubMed]
- Sutter R, Kaplan PW. Electroencephalographic criteria for nonconvulsive status epilepticus: synopsis and comprehensive survey. Epilepsia 2012;53 Suppl 3:1-51. [Crossref] [PubMed]
- Maganti R, Gerber P, Drees C, Chung S. Nonconvulsive status epilepticus. Epilepsy Behav 2008;12:572-86. [Crossref] [PubMed]
- Kurtz P, Gaspard N, Wahl AS, Bauer RM, Hirsch LJ, Wunsch H, Claassen J. Continuous electroencephalography in a surgical intensive care unit. Intensive Care Med 2014;40:228-34. [Crossref] [PubMed]
- Privitera M, Hoffman M, Moore JL, Jester D. EEG detection of nontonic-clonic status epilepticus in patients with altered consciousness. Epilepsy Res 1994;18:155-66. [Crossref] [PubMed]


