Multi-detector CT angiography of the aortic valve—Part 1: anatomy, technique and systematic approach to interpretation
Introduction
The aortic valve can be affected by a wide range of both congenital and acquired diseases. Echocardiography and magnetic resonance imaging (MRI) are the main imaging techniques used for assessment of the aortic valve and related pathology but multi-detector computed tomography (MDCT) can offer valuable complimentary information in certain clinical scenarios. Radiologists should be familiar with the role of MDCT in this setting in order to advise and direct an appropriate imaging strategy depending upon the clinical question. The aortic valve and adjacent structures should also be routinely evaluated on all thoracic MDCT cross sectional studies, regardless of clinical indication, as aneurysms and other undiagnosed pathology may be first identified on such examinations. We present a 2-part review concerning the use of MDCT in aortic valve assessment. This article, Part 1 of the review focuses on aortic valve anatomy, MDCT imaging protocols, reconstruction techniques and a systematic approach to interpretation. Part 2 covers the key MDCT imaging features of the more common diseases to involve the aortic valve and its support structures.
Normal anatomy
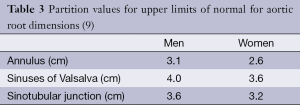
The aortic root forms a bridge between the left ventricular outflow tract (LVOT) and ascending aorta (Ao), extending from the aortic valve annulus to the sinotubular junction which is a circumferential indentation composed primarily of elastic tissue (1). The walls of the aortic root are formed by three focal expansions called the sinuses of Valsalva which are hollow spaces bounded medially by the aortic valve leaflets (Figure 1). The normal aortic valve consists of three leaflets, each approximately 1 mm thick with ventricular and arterial surfaces lined by endothelial cells. The sinuses provide space behind the valve leaflets when open so that they do not occlude the coronary ostia and help reduce damage to the valve leaflets as a result of striking the aortic root wall (2). During normal valve opening, the leaflets retract to form a triangular-shaped orifice and with normal valve closure the leaflets should completely meet (coapt) centrally.
The coronary ostia arise from the aortic root wall, just above the sinuses with the left main stem artery arising just above the left sinus and the right coronary artery just above the right sinus (1). The non-coronary (NC) sinus is sited posteriorly. The sinuses are intracardiac and intimately related to the cardiac chambers and pulmonary outflow tract. The right coronary sinus lies adjacent to the pulmonary outflow tract, interventricular septum and right atrium (2). The NC sinus is adherent to the interatrial septum and adjacent to the atrioventricular node and His bundle. The left coronary sinus is adherent to the anterior wall of the left atrium (LA) (2).
Non-invasive imaging techniques
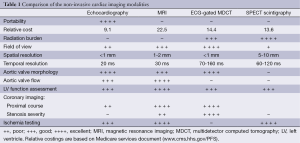
Transthoracic echocardiography (TTE) is the current imaging standard for assessment of the aortic valve. In many patients TTE can provide sufficient information to complete the diagnostic evaluation but when assessment is incomplete or inconclusive other diagnostic techniques must then be considered depending upon the clinical question. The relative strengths and weakness of the respective non-invasive imaging modalities in providing assessment of various aspects of an aortic valve study are summarised in Table 1.
Full table
Echocardiography
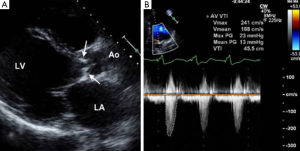
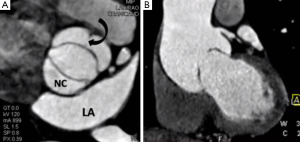
Provided acoustic windows are adequate the aortic valve and related structures can be well visualised with TTE (Figure 2). TTE is cost effective, portable and widely accessible, and in many cases will provide sufficient information to establish a diagnosis and guide treatment decisions. TTE is a real-time examination with excellent temporal resolution (20-30 msec) and can also provide reliable estimations of transvalvular pressure gradients. The main disadvantages of TTE are operator dependence, and a rather limited field of view, especially in patients with emphysema in whom acoustic windows may be severely restricted. Transoesophageal echocardiography (TOE) enables a transducer to be placed in close approximation to the heart which gives improved spatial resolution over TTE including much better identification of endocarditis vegetations (3). TOE is however an invasive procedure (requiring sedation/general anaesthesia) that is not always tolerated or without risk and as with TTE, cannot interrogate the entire thoracic Ao. If echocardiography assessment is incomplete or inconclusive MRI and/or MDCT may be considered depending upon the clinical scenario (4).
MRI
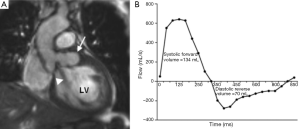
MRI can provide a comprehensive evaluation of anatomy, transvalvular flow and left ventricular function. It is not dependent on acoustic windows and is less reliant than TTE on operator experience. MRI has good temporal resolution (20-40 msec) which is sufficient to assess both myocardial and valve motion. Phase-contrast sequences provide consistent, reproducible measurement of blood flow and can accurately quantify severity of aortic valve stenosis and/or regurgitation (Figure 3) (5). Current MRI limitations are its relatively low spatial resolution (1-2 mm), prolonged examination time (45 minutes for a typical combined aortic and structural cardiac assessment) and its contraindications e.g., those with an implanted pacemaker device or cerebral aneurysm clips. Prosthetic valves can be safely imaged but there is often significant image artefact which may preclude detailed assessment of component function. Finally MRI may not be tolerated in patients with claustrophobia.
MDCT
ECG-gated MDCT provides isotropic high spatial resolution images (0.4-0.6 mm) with an unrestricted field of view that can be acquired in a single breath-hold enabling non-invasive coronary artery assessment. Volume coverage is unlimited allowing evaluation of the aortic arch to iliac vessels in a single study and the ability to obtain multi-planar reformations in any desired plane from the source dataset effectively eliminates operator dependence (Figure 4). MDCT is the most sensitive technique for detecting calcification of the aortic valve and aortic wall, important for surgical planning. Calcium scoring of aortic valve, even when asymptomatic, has been shown useful to predict short-term clinical outcome and the need for further surveillance or early valve replacement (4).
MDCT is also the most accurate and reproducible method for measuring the aortic annulus dimensions which is a critical part of trans-catheter aortic valve implantation (TAVI) planning (6). Aortic valve motion and left ventricular wall motion can be assessed with MDCT but requires the use of retrospective ECG-gating (continuous data acquisition through the cardiac cycle) which has a high radiation exposure, especially for coverage of the entire thoracic Ao (10-20 mSv). Other current limitations of MDCT are its relatively low temporal resolution (75-180 msec) and inability to directly measure the transvalvular flow velocity.
MDCT technique
The aortic valve is included on a standard coronary artery imaging protocol (covering from carina to cardiac apex), but the aortic arch and great vessels are not. Some pathology affecting the aortic valve may originate in (e.g., aortic dissection) or secondarily involve the thoracic Ao (e.g., bicuspid valve associated aortopathy or coarctation). Aortic root pathology can also affect the left ventricle (LV) (e.g., LV hypertrophy with aortic stenosis). Hence the exact MDCT protocol used should be tailored to answer the clinical question (Table 2). Intravenous contrast agent is administered via a right arm peripheral 18 gauge cannula via a pump at 5 mL/s. The scan is initiated by bolus tracking on the descending Ao triggered at 180 HU. The imaged section thickness is 0.6-1.0 mm.

Full table
ECG-gating reduces cardiac motion artefacts, which enables non-invasive coronary artery evaluation and cardiac morphological assessment. ECG-gating can be achieved using a retrospective or prospective technique. Retrospective imaging acquires continuous data through the cardiac cycle allowing the dataset to be run in cine mode for myocardial and valvular motion assessment. Prospective imaging acquires data at a preselected diastolic phase of the cardiac cycle, and thus no functional evaluation can be performed. The prospective approach carries a much lower radiation burden but requires a regular and slow heart rhythm (<70 beats per minute and <10% beat to beat variability) to avoid significant image artefacts.
Systematic approach to MDCT interpretation
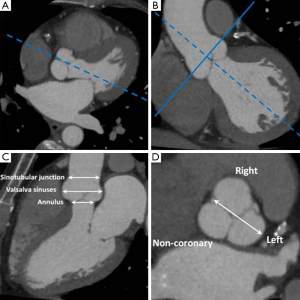
Aortic root dimensions are conventionally measured at three levels: (I) aortic valve annulus (the hinge points of the valve cusps); (II) midpoint of the sinuses of Valsalva; and (III) sinotubular junction. Measurements are conventionally made from an “optimised” sagittal oblique LVOT reconstruction in the mid-diastolic (prospective ECG-gating) or end-diastolic (retrospective ECG-gating) phase (7). In order to achieve this reconstruction plane from an MDCT dataset a line transecting the mid-point of the aortic valve and LVOT is first selected (Figure 5). The resultant image is a coronal LVOT plane through which a plane transecting the LVOT and LV apex is selected to create an “off-set” sagittal oblique image. This plane is then carefully adjusted so that the mid-aortic valve, mid-mitral valve and LV apex are all aligned to produce an “optimised” sagittal oblique LVOT image. This MDCT plane is analogous to the parasternal long axis view on echocardiography from which much of the normative aortic root data is derived. A line perpendicular to the Valsalva sinuses taken from the “optimised” LVOT view will provide a true cross-sectional plane through the aortic valve leaflets and this plane has been shown as the most reproducible for measurement of aortic root dimensions (8). The ability to measure aortic root dimensions using MDCT which provides an unrestricted imaging plane is a particular advantage over TTE which may be limited by acoustic windows and therefore prone to measurement error. Indeed a recent study showed TTE measurements to be substantially lower or even normal in patients found to have a dilated aortic root by MDCT assessment (8). Aortic dimensions vary according to gender and body habitus but upper limits of normal have been defined and form a useful reference guide (Table 3) (9).
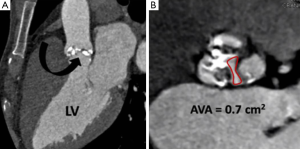
Once aortic root dimensions have been assessed valve morphology is examined. The number of leaflets is documented (tricuspid, bicuspid etc.) and the presence of valvular calcification and free edge thickening is noted (10). On retrospective ECG-gated studies leaflet excursion is studied on a cine loop reconstruction in the cross sectional valve plane. Absence of normal central coaptation (suggestive of aortic regurgitation) or the presence of leaflet prolapse is specifically looked for. If there is felt to be a restricted opening pattern the AVA is planimetered using the end-systolic frame (Figure 6) (11).
Next the coronary artery origins are assessed by documenting the sinus of origin, proximal course of each vessel and the presence of any anomalous vessels. The LV should also be routinely evaluated for wall thickness and cavity dimension (measuring in the diastolic phase). Upper limits of normal for diastolic left ventricular wall thickness are 12 mm within the septum and 8 mm for the lateral wall. Diastolic cavity dimension should be <6 cm as a general guide (12). Ejection fraction should be calculated on retrospective datasets—this can usually be achieved with vendor specific contouring algorithms to map the endocardial borders and measure end-diastolic and end-systolic volumes. Finally the thoracic Ao is evaluated, documenting the ascending, arch and descending dimensions. Aneurysms and aortic coarctation are specifically looked for.
Conclusions
This section completes Part 1 of this 2-part review on MDCT evaluation of the aortic valve. Relevant anatomy has been reviewed and MDCT protocols have been discussed. In addition reconstruction techniques and an approach to interpretation have been described. Part 2 of this review deals with the MDCT features of the more common disease processes to involve the aortic valve and its support structures.
Disclosure: The authors declare no conflict of interest.
References
- Underwood MJ, El Khoury G, Deronck D, Glineur D, Dion R. The aortic root: structure, function, and surgical reconstruction. Heart 2000;83:376-80. [PubMed]
- Anderson RH. Clinical anatomy of the aortic root. Heart 2000;84:670-3. [PubMed]
- Baddour LM, Wilson WR, Bayer AS, Fowler VG Jr, Bolger AF, Levison ME, Ferrieri P, Gerber MA, Tani LY, Gewitz MH, Tong DC, Steckelberg JM, Baltimore RS, Shulman ST, Burns JC, Falace DA, Newburger JW, Pallasch TJ, Takahashi M, Taubert KA; Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease; Council on Cardiovascular Disease in the Young; Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia; American Heart Association; Infectious Diseases Society of America. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 2005;111:e394-434. [PubMed]
- Gilkeson RC, Markowitz AH, Balgude A, Sachs PB. MDCT evaluation of aortic valvular disease. AJR Am J Roentgenol 2006;186:350-60. [PubMed]
- Maganti K, Rigolin VH, Sarano ME, Bonow RO. Valvular heart disease: diagnosis and management. Mayo Clin Proc 2010;85:483-500. [PubMed]
- Binder RK, Webb JG, Willson AB, Urena M, Hansson NC, Norgaard BL, Pibarot P, Barbanti M, Larose E, Freeman M, Dumont E, Thompson C, Wheeler M, Moss RR, Yang TH, Pasian S, Hague CJ, Nguyen G, Raju R, Toggweiler S, Min JK, Wood DA, Rodés-Cabau J, Leipsic J. The impact of integration of a multidetector computed tomography annulus area sizing algorithm on outcomes of transcatheter aortic valve replacement: a prospective, multicenter, controlled trial. J Am Coll Cardiol 2013;62:431-8. [PubMed]
- Burman ED, Keegan J, Kilner PJ. Aortic root measurement by cardiovascular magnetic resonance: specification of planes and lines of measurement and corresponding normal values. Circ Cardiovasc Imaging 2008;1:104-13. [PubMed]
- Ocak I, Lacomis JM, Deible CR, Pealer K, Parag Y, Knollmann F. The aortic root: comparison of measurements from ECG-gated CT angiography with transthoracic echocardiography. J Thorac Imaging 2009;24:223-6. [PubMed]
- Roman MJ, Devereux RB, Kramer-Fox R, O'Loughlin J. Two-dimensional echocardiographic aortic root dimensions in normal children and adults. Am J Cardiol 1989;64:507-12. [PubMed]
- Bennett CJ, Maleszewski JJ, Araoz PA. CT and MR imaging of the aortic valve: radiologic-pathologic correlation. Radiographics 2012;32:1399-420. [PubMed]
- Hoey ET, Ganeshan A, Nadar SK, Gulati GS. Evaluation of the aortic root with MRI and MDCT angiography: spectrum of disease findings. AJR Am J Roentgenol 2012;199:W175-86. [PubMed]
- Boxt LM, Lipton MJ, Kwong RY, Rybicki F, Clouse ME. Computed tomography for assessment of cardiac chambers, valves, myocardium and pericardium. Cardiol Clin 2003;21:561-85. [PubMed]