Multi-detector CT angiography of the aortic valve—Part 2: disease specific findings
Introduction
The aortic valve can be affected by a wide range of both congenital and acquired diseases. Echocardiography and magnetic resonance imaging (MRI) are the main imaging techniques used for assessment of the aortic valve and related pathology but multi-detector computed tomography (MDCT) can offer valuable complimentary information in certain clinical scenarios. Radiologists should be familiar with the role of MDCT in this setting in order to advise and direct an appropriate imaging strategy depending upon the clinical question. The aortic valve and adjacent structures should also be routinely evaluated on all thoracic MDCT cross sectional studies, regardless of clinical indication, as aneurysms and other undiagnosed pathology may be first identified on such examinations. We present a 2-part review concerning the use of MDCT in aortic valve assessment. Part 1 of the review focuses on aortic valve anatomy, MDCT imaging protocols, reconstruction techniques and a systematic approach to interpretation. This article, part 2 covers the key MDCT imaging features of the more common diseases to involve the aortic valve and its support structures.
Congenital leaflet anomalies
Bicuspid aortic valve (BAV)
BAV affects 1-2% of the population and is the most common congenital cardiac defect (1). BAV is hereditary (autosomal dominant) with incomplete penetrance and a strong male predominance (3:1). It has a strong association with other cardiac and vascular malformations, particularly aortic coarctation which is present in around 25% of patients with BAV (2,3). BAV is also associated with progressive dilatation of the aortic root and ascending thoracic aorta (termed BAV aortopathy) which has been attributed to an abnormal connective tissue matrix in conjunction with abnormal helical flow patterns generated by the valve morphology. Aortopathy is typically progressive with patients requiring serial imaging assessment regards timing of aortic valve and root replacement surgery with an aortic diameter of 5 cm used as guide for potential intervention (1).
Most commonly a BAV is composed of two leaflets of unequal size. The larger leaflet is usually the result of congenital fusion in which case a fusion line (termed a “raphe”) is often visible both at gross inspection and at MDCT angiography. Rarely no raphe is identified and this is termed a “pure” BAV (4). The commonest pattern of leaflet fusion is left and right (70% of BAV) followed by right and non-coronary leaflets (28%). Fusion of the left and non-coronary leaflets is rare (2%) (4). A BAV undergoes premature degeneration which is thought to be multifactorial with increased sheer stress as a major contributor. Indeed BAV is the commonest cause of aortic stenosis (AS) and regurgitation in the under 65-year age group.
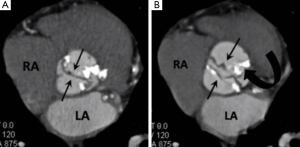
MDCT has been shown as an accurate means of diagnosing BAV with comparable results to MRI and TTE however its particular utility is in the setting of BAV degeneration when heavy leaflet calcification artefact may limit the accuracy of MRI and TTE (5). A BAV is best seen in systole when it has a “fishmouth” appearance which describes the classical elliptical shaped orifice in contrast to the triangular orifice of a normal trileaflet aortic valve (6). When a BAV undergoes degeneration calcification tends to predominate along the fusion line and the base of the conjoined leaflets (Figure 1) (6).
Quadricuspid aortic valve
A quadricuspid (4-leaflet) aortic valve is extremely rare. As with BAV there is a strong association with premature valvopathy and aortopathy but these complications typically present at an earlier age than with BAV. Quadricupsid valves are particularly associated with aortic regurgitation (AR) (75%) and nearly 20% of affected patients have other co-existing congenital cardiac abnormalities (such as single coronary artery) which should be sought. There are only a handful of reports describing the MDCT features but typically the 4th leaflet is small and redundant (5,6).
Aortic stenosis (AS)
AS describes obstruction of left ventricular outflow at the level of the valve with a pressure gradient of greater than 10 mmHg (7). It most commonly occurs secondary to calcific degeneration of a trileaflet valve (usually in a patient >65 years) or complicating a BAV (usually in a patient <65 years). Valve calcification was initially thought to be a chronic degenerative process, but has recently been shown to have an active inflammatory component with many similarities to atherosclerosis. Calcific aortic valve degeneration begins at the bases of the valve leaflets before spreading to involve the leaflet margins, leading to a reduction of the aortic valve area (AVA) and restricted leaflet movement (8). Typically the non-coronary leaflet is affected first. Reduced AVA leads to increasing pressure gradients between the valve and ascending aorta, which is used to grade AS severity as mild (<25 mmHg/AVA >1.5 cm2), moderate (25-40 mmHg/AVA 1.5-1 cm2) and severe (>40 mmHg/AVA <1 cm2) (7).
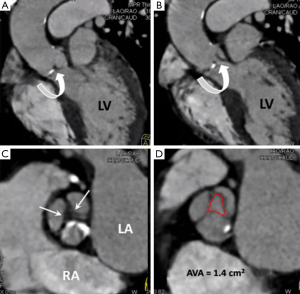
MDCT features of AS are leaflet thickening and calcifications, decreased valvular excursion (requires a retrospective scan protocol), post stenotic aortic dilatation and concentric left ventricular hypertrophy. MDCT relies on AVA planimetry in end systole (20% R-R interval or 50-100 ms from the R wave peak) to infer stenosis severity (7). This method, although seldom used in day to day clinical practice has been shown to correlate well with TTE derived stenosis severity (Figure 2). MDCT derived severity assessment of leaflet calcification, has been shown to have good correlation with stenosis severity as assessed by TTE and serves as a useful guide when valve calcification is seen on routine thoracic cross-sectional MDCT (8):
- Grade 1, no calcification;
- Grade 2, mildly calcified (small isolated spots);
- Grade 3, moderately calcified (multiple larger spots);
- Grade 4, heavily calcified (extensive calcification on all cusps).
One of the main roles of MDCT in the setting of AS is as a key imaging technique in the work-up of patients with severe AS being considered for transcatheter aortic valve implantation (TAVI).
Aortic valve replacement
Valve replacement is the treatment of choice for patients with severe symptomatic AS (9). Preoperative assessment requires measurement of the aortic valve annulus and thoracic aortic dimensions as well as evaluation of left ventricular ejection fraction and coronary artery anatomy. MDCT can provide a “one-stop”, comprehensive assessment of these variables and has an increasing role in this setting (7).
Up to 40% of patients with severe AS may not be suitable for valve replacement surgery due to co-morbidities and high anaesthetic risk and there is an increasing interest in the role of TAVI in this patient group. Since its first description in 2002 (TAVI) has become established as a viable alternative to surgical aortic valve replacement in AS patients ineligible for surgery. TAVI involves valvulolasty of the native diseased valve to displace the leaflets followed by catheter delivery of a valve prosthesis mounted on a metallic stent (10). Intra-procedural prosthesis positioning is confirmed with fluoroscopy and transesophageal echocardiography (TEE). A transfemoral approach is preferred but subclavian, axillary and transapical techniques have also been described (10).
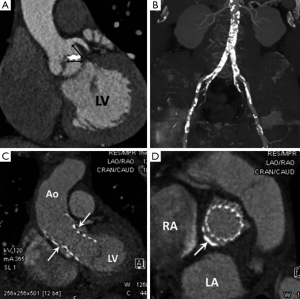
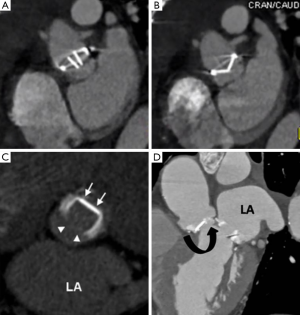
Pre-procedural TAVI work-up requires accurate measurement of aortic valve annulus dimensions to avoid anulo-prosthesis mismatch (which can lead to stent migration and/or paravalvular leakage). MDCT is considered the “gold-standard” for annulus measurements which are often underestimated by TEE due to its elliptical shape (10). MDCT is also used to measure the distance between the annulus and coronary artery ostia (to avoid coverage during valvuloplasty) and to map the abdominal aorta and iliofemoral arteries for tortuosity, stenoses and heavy calcific plaque burden which can be a contraindication to a transfemoral route (Figure 3A,B). For transfemoral TAVI a minimal arterial lumen diameter of 7 mm is required. Acute angulation and protruding atheroma (which might embolise with catheter manipulation) are also considered relative contraindications and may necessitate an alternative approach (10). A summary of key MDCT components of a TAVI study are presented in Table 1. There is also an increasing use of MDCT for post-operative TAVI follow-up (Figure 3C,D).
Full table
Aortic regurgitation (AR)
AR describes insufficient leaflet coaptation in diastole which causes diastolic backflow of blood from the ascending aorta into the left ventricle (LV). AR can occur secondary to congenital or acquired leaflet dysfunction, aortic annulus dilatation or a combination of the two (11). Globally valve damage from rheumatic fever is the commonest cause of AR, but in Western populations BAV disease and aortic root dilatation are the commonest causes (12). AR causes a progressive increase in left ventricular volumes and pressure. If the AR is acute (e.g., associated with acute aortic dissection), acute pulmonary edema may develop as the LV fails to adapt to a sudden increase in filling pressure. With chronic AR the volume loading is more gradual which allows for remodelling with eccentric LV hypertrophy and chamber dilatation. The need for surgical valve replacement depends upon symptom severity, regurgitant fraction (>30%), LV function (ejection fraction <50%) and LV cavity size (diastolic diameter >7 cm) (11-13). Accurate sizing of the aortic root and thoracic aortic dimensions are important to guide the surgical repair technique (11). Echocardiography remains the first line tool for assessment of AR severity and LV size and function however it may struggle to accurately size the ascending aorta for which MDCT is well suited.
The aetiology of AR can be suggested with MDCT by evaluating aortic dimensions in conjunction with leaflet thickness and calcification. With intrinsic valve disease, the leaflets are often retracted, thickened and calcified. When AR is secondary to aortic root distortion the leaflets may have a normal appearance but fail to coapt centrally leaving a diastolic phase regurgitant orifice (11-13). Leaflet malcoaptation is best visualized with MDCT in the end diastolic phase of the cardiac cycle with images reconstructed at approximately 70% of the R-R interval (approximately 600 msec from the R-wave peak) (11). AVA planimetry can be used to quantitatively assess AR severity with several studies showing a good correlation with TTE for moderate and severe AR although this technique is rarely used in clinical practice (12,13).
Infective endocarditis (IE)
IE is a potentially life-threatening condition that frequently involves the aortic valve with streptococci and staphylococci account for around 80% of cases (14). Major risk factors for IE are congenital structural anomalies such as BAV, prosthetic valves and intravenous drug use. Bacterial endocarditis causes rapid leaflet destruction, perforation and severe AR. Infection commonly spreads into the periannular soft tissues and can cause abscesses, pseudoaneurysms and fistulas with the incidence of these complications reported to be 30-50% for aortic valve IE (15). There is also an increased risk of stroke, mycotic intracerebral abscess and splenic abscess formation secondary to systemic embolization.
Treatment of IE is usually with prolonged courses of intravenous antibiotics but surgical resection and valve replacement may be considered in the setting of severe AR, large valvular vegetations and perivalvular pseudoaneurysm/abscess or fistula (16). Hence the preoperative recognition of an infected pseudoaneurysm has important therapeutic implications requiring much more extensive surgical intervention with the need for debridement and often annulus reconstruction. Diagnosis of IE is established using the revised Duke criteria which are a collection of major (positive blood culture with typical IE microorganism, evidence of endocardial involvement at echocardiography) and minor [predisposing factor for IE, evidence of systemic or pulmonary emboli (e.g., splenic infarct) and immunological conditions such as glomerulonephritis] criteria. The hallmark imaging sign of endocarditis are vegetations which are often small (<5 mm) and can be difficult to detect with TTE which has a sensitivity of only 25% for small vegetations (14). TEE has a reported sensitivity of 48-100% for small vegetations and is considered the reference imaging technique (14). Perivalular pseudoaneurysms can however be very difficult to define with TTE and TEE due to limited soft tissue resolution and MDCT has an emerging role in this setting. Feuchtner et al. showed a high sensitivity (96%) and specificity (97%) for MDCT detection of leaflet vegetations >4 mm and significantly improved accuracy of MDCT over TEE for detection and localization of perivalvular abscesses and pseudoaneyrms (15). One other potential advantage of MDCT is non-invasive assessment of coronary artery disease, as catheter directed coronary angiography carries a high risk of vegetation embolisation with catheter manipulation (15). On MDCT a vegetation appears as a low attenuation lesion adherent to the valve surface. An abscess appears as a peripherally enhancing mass which is typically located within the periannular soft tissues but can also involve the myocardium or even extend into the pericardial space. An infected pseudoaneurysm appears as a contrast medium filling lesion in direct communication with the aortic root or LVOT (Figure 4) (15,16).
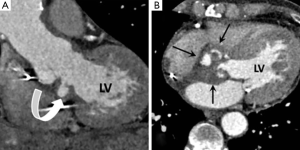
Tumours
Papillary fibroelastoma is the commonest heart valve tumour and most frequently involves the aortic valve (17). They are histologically benign being composed of a meshwork of collagen and elastic fibres covered by a thin endothelial lining (17,18). Up to 50% are discovered incidentally at TTE performed for another indication although there is a potential risk for systemic embolisation of tumour fragments or accumulated thrombus (19). Fibroelastomas do not usually interfere with valve function. MDCT features are of a small (usually <1 cm) well circumscribed low attenuation nodule which often has a pedicle (Figure 5) (20). The most common location on the aortic valve is its upstream side (17). A vegetation of IE is the main imaging differential diagnosis but distinction is usually straightforward on clinical grounds and vegetations are invariably associated with severe AR (20).
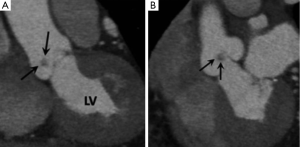
Prosthetic valve complications
MDCT does not currently form part of the routine assessment of patients with suspected mechanical valve dysfunction and is usually reserved for selected cases for which information on valve size and function cannot be obtained by cine fluoroscopy or TTE/TEE. Mechanical heart valves typically cause less image artifact with MDCT compared with MRI and echocardiography and several recent studies have shown it to be an accurate means of measuring prosthetic valve opening angle, annulus diameter, and geometric orifice area which are essential in the assessment of prosthetic valve dysfunction (Figure 6A,B) (21). Soft tissue ingrowth or thrombus can cause a “stuck” leaflet with resultant valvular stenosis (Figure 6C). A paravalvular leak can occur if the prosthesis ring separates away from the native annulus (Figure 6D).
Conclusions
This section completes Part 2 of this 2-part review on MDCT evaluation of the aortic valve. The most common disease processes to involve the aortic valve have been discussed and illustrated with a particular emphasis on MDCT features. Radiologists should be familiar with these disease processes and their key imaging features to enable both accurate diagnoses and relevant additional information.
Disclosure: The authors declare no conflict of interest.
References
- Garg V. Molecular genetics of aortic valve disease. Curr Opin Cardiol 2006;21:180-4. [PubMed]
- Huntington K, Hunter AG, Chan KL. A prospective study to assess the frequency of familial clustering of congenital bicuspid aortic valve. J Am Coll Cardiol 1997;30:1809-12. [PubMed]
- Tawes RL Jr, Berry CL, Aberdeen E. Congenital bicuspid aortic valves associated with coarctation of the aorta in children. Br Heart J 1969;31:127-8. [PubMed]
- Sabet HY, Edwards WD, Tazelaar HD, Daly RC. Congenitally bicuspid aortic valves: a surgical pathology study of 542 cases (1991 through 1996) and a literature review of 2,715 additional cases. Mayo Clin Proc 1999;74:14-26. [PubMed]
- Gilkeson RC, Markowitz AH, Balgude A, Sachs PB. MDCT evaluation of aortic valvular disease. AJR Am J Roentgenol 2006;186:350-60. [PubMed]
- Ryan R, Abbara S, Colen RR, Arnous S, Quinn M, Cury RC, Dodd JD. Cardiac valve disease: spectrum of findings on cardiac 64-MDCT. AJR Am J Roentgenol 2008;190:W294-303. [PubMed]
- Laissy JP, Messika-Zeitoun D, Serfaty JM, Sebban V, Schouman-Claeys E, Iung B, Vahanian A. Comprehensive evaluation of preoperative patients with aortic valve stenosis: usefulness of cardiac multidetector computed tomography. Heart 2007;93:1121-5. [PubMed]
- Koos R, Mahnken AH, Sinha AM, Wildberger JE, Hoffmann R, Kühl HP. Aortic valve calcification as a marker for aortic stenosis severity: assessment on 16-MDCT. AJR Am J Roentgenol 2004;183:1813-8. [PubMed]
- Schwarz F, Baumann P, Manthey J, Hoffmann M, Schuler G, Mehmel HC, Schmitz W, Kübler W. The effect of aortic valve replacement on survival. Circulation 1982;66:1105-10. [PubMed]
- Webb JG, Pasupati S, Humphries K, Thompson C, Altwegg L, Moss R, Sinhal A, Carere RG, Munt B, Ricci D, Ye J, Cheung A, Lichtenstein SV. Percutaneous transarterial aortic valve replacement in selected high-risk patients with aortic stenosis. Circulation 2007;116:755-63. [PubMed]
- Alkadhi H, Desbiolles L, Husmann L, Plass A, Leschka S, Scheffel H, Vachenauer R, Schepis T, Gaemperli O, Flohr TG, Genoni M, Marincek B, Jenni R, Kaufmann PA, Frauenfelder T. Aortic regurgitation: assessment with 64-section CT. Radiology 2007;245:111-21. [PubMed]
- Feuchtner GM, Dichtl W, Schachner T, Müller S, Mallouhi A, Friedrich GJ, Nedden DZ. Diagnostic performance of MDCT for detecting aortic valve regurgitation. AJR Am J Roentgenol 2006;186:1676-81. [PubMed]
- Jassal DS, Shapiro MD, Neilan TG, Chaithiraphan V, Ferencik M, Teague SD, Brady TJ, Isselbacher EM, Cury RC. 64-slice multidetector computed tomography (MDCT) for detection of aortic regurgitation and quantification of severity. Invest Radiol 2007;42:507-12. [PubMed]
- Mylonakis E, Calderwood SB. Infective endocarditis in adults. N Engl J Med 2001;345:1318-30. [PubMed]
- Feuchtner GM, Stolzmann P, Dichtl W, Schertler T, Bonatti J, Scheffel H, Mueller S, Plass A, Mueller L, Bartel T, Wolf F, Alkadhi H. Multislice computed tomography in infective endocarditis: comparison with transesophageal echocardiography and intraoperative findings. J Am Coll Cardiol 2009;53:436-44. [PubMed]
- Gahide G, Bommart S, Demaria R, Sportouch C, Dambia H, Albat B, Vernhet-Kovacsik H. Preoperative evaluation in aortic endocarditis: findings on cardiac CT. AJR Am J Roentgenol 2010;194:574-8. [PubMed]
- Gowda RM, Khan IA, Nair CK, Mehta NJ, Vasavada BC, Sacchi TJ. Cardiac papillary fibroelastoma: a comprehensive analysis of 725 cases. Am Heart J 2003;146:404-10. [PubMed]
- Grinda JM, Couetil JP, Chauvaud S, D’Attellis N, Berrebi A, Fabiani JN, Deloche A, Carpentier A. Cardiac valve papillary fibroelastoma: surgical excision for revealed or potential embolization. J Thorac Cardiovasc Surg 1999;117:106-10. [PubMed]
- Eftekhari H, Islam A, Slawsky M. Aortic valve fibroelastoma presenting with myocardial infarction. Catheter Cardiovasc Interv 2011;77:716-9. [PubMed]
- Sparrow PJ, Kurian JB, Jones TR, Sivananthan MU. MR imaging of cardiac tumors. Radiographics 2005;25:1255-76. [PubMed]
- Hoang JK, Martinez S, Hurwitz LM. MDCT angiography after open thoracic aortic surgery: pearls and pitfalls. AJR Am J Roentgenol 2009;192:W20-7. [PubMed]


