
Altered intrinsic functional connectivity of the primary visual cortex in patients with retinal vein occlusion: a resting-state fMRI study
Introduction
Retinal vein occlusion (RVO) is a general retinal vascular disease with latent threatening to vision, which can be categorized into branch RVO and central RVO contingent on the affected vein obstruction (1). Previous epidemiological studies reveal that RVO is the 2nd frequent retinal vessel disorder following diabetic retinopathy leading to visual impairment and the prevalence vary from 0.5% to 2.1% of the middle age and old age group (2-4). RVO generally occurs unilaterally and exhibits diverse variations on the degrees of severity, which is ordinarily characterized by deep and superficial intraretinal haemorrhages, varying degrees of retinal venous congestion and tortuosity, cotton wool spots and cystoid macular oedema (5). Retinal ischemia, which might be complicated by RVO, often lead to a poor prognosis and potentially promote other complications (6-8), such as vitreous hemorrhage, retinal neovascularization, and neovascular glaucoma. The systemic risk factors of RVO vary from typical atherosclerosis to others as hypertension, diabetes mellitus, dyslipidemia and cerebral stroke, and the main treatments for RVO include the laser, steroid, and anti-VEGF (9,10). Routine ocular examinations for RVO include fundus fluorescein angiography (FFA) and optical coherence tomography (OCT) (11), which simply focus on the alterations of fundus itself, while the neuroimaging detection remain rarely. The investigation of RVO-related brain processes through neuroimaging is a new aspect in visual neuroscience which may help to reveal the potential mechanisms (12,13).
Resting-state functional magnetic resonance imaging (rs-fMRI), a noninvasive neuroimaging technique depend on analyzing cerebral blood flow and metabolism, has been developed increasingly and enabled researchers to inspect functional alterations of specific brain area at rest state (14). Among those analysis methods used for rs-fMRI, seed-based functional connectivity (FC) analysis where the correlation coefficients between the average blood oxygen level-dependent (BOLD) time series of a predetermined cerebral area and that of each voxel or other pre-selected seeds in the brain are calculated, is the most extensively used technique on account of its sensitive, straightforward, and easily elucidated to provide insights into neural interactions (15). The FC analysis has been successfully used for discovering the intrinsic neural changes in some visual-related diseases, and many recent studies have demonstrated that abnormal spontaneous FC were observed in patients with glaucoma (16), amblyopia (17) and strabismus (18,19) between the primary visual cortex (V1) and the other cortex. V1 is the kernel of the visual network, located in the occipital lobe (20). It receives visual input directly from the lateral geniculate nucleus, encodes visual information and then transmits to the senior visual cortex to develop complicated functional connections (21). However, the intrinsic FC alterations of V1 in RVO has not been revealed yet.
Therefore, our present study proposed to use the seed-based FC method to obtain the FC between V1 and the other cortical regions, which might provide a more accurate and exhaustive view on the specific connectivity of V1 in RVO, further help to understand the underlying neural mechanisms of impaired visual function in RVO patients comparing with healthy controls (HCs).
Methods
Participants
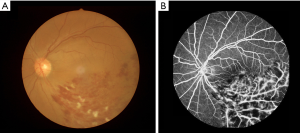
Twenty-one subjects with RVO (11 males, 10 females) were enlisted with the following inclusion criteria in the First Affiliated Hospital of Nanchang University from 2017.03.01 to 2018.09.30: (I) ophthalmoscopy showed signs of RVO; (II) OCT showed macular oedema; (III) FFA showed occlusion of retinal vein (Figure 1). The exclusion criteria of RVO were: (I) patients with prior intraocular surgery or extraocular surgery history; (II) complicated with other ocular diseases; (III) neurological diseases, psychiatric disorders, cardiovascular diseases and other systematic diseases.
Twenty-one HCs (11 males, 10 females) comparably matched in age and sex background with RVO group were enrolled. Inclusion criteria were: (I) no ocular disease history; (II) no deformities in the cerebral parenchyma; (III) no neurological diseases, psychiatric diseases and cardiovascular diseases; (IV) no drugs or alcohol addiction; (V) capable of MRI examination.
All research methods were approved by the Medical Ethics Committee of the First Affiliated Hospital of Nanchang University (CDYFY-LL-2017025) and complied with the Helsinki Declaration. The whole study was carried out in accordance with the approved guidelines. All participants were notified the objectives, contents, latent risks, and signed informed consent agreements for this study.
MRI parameters
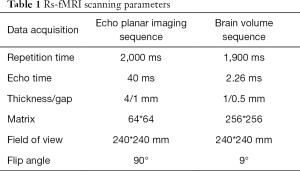
Rs-fMRI was scanned with a 3-Tesla MR scanner (Siemens, Munich, Germany). The total scanning time of data acquisition was 8 minutes. The whole-brain high-resolution T1-weighted images of each participants were obtained by using a 3D spoiled gradient-recalled echo sequence with the detailed parameters shown in Table 1.
Full table
FMRI data processing
We preprocessed data by applying SPM8 (http://www.fil.ion.ucl.ac.uk/spm), DPARSFA (http://rfmri.org/DPARSF) and Static State Data Analysis Toolkit (REST, http://www.restfmri.net). After deleting the first 10 time points, the remaining 230 volumes were to be collected. The main preprocessing procedures were as follows: (I) slice timing; (II) co-registration of functional images to high-resolution T1-weighted structural images, spatial normalization to standard Montreal Neurological Institute (MNI) space; (III) smoothing with a 6 mm full-width-half-maximum Gaussian kernel; (IV) filtering (0.01±0.1 Hz) to reduce the effects of low-frequency drifts and high-frequency noise; (V) regressing out head motion as well as white matter, cerebrospinal fluid and global signals as nuisance variables. The participants that volumes with the x, y, or z directions more than 2 were excluded from this study. The previous study prescribed more details (16,18).
Seed-based functional connectivity analysis
Region of interest (ROI) was preselected in accordance with previous literature (16,22). The V1, also known as Broadmann area 17 (BA 17) was predefined as the seed point, the MNI coordinates were left V1 (−8, −76, 10) and right V1 (+7, −76, 10) respectively. The diameter of the circular ROI was 10 mm, and Pearson correlation coefficients were computed with Fisher’s z transform analysis between the mean time series of the ROI and the time series of other cerebrum voxels.
Statistical analysis
SPSS ver. 20.0 (SPSS, IBM Corp, USA) was applied to compare the demographic and clinical data between RVOs and HCs with independent sample t-test (age and weight) and Chi-square test (gender and handedness), and P<0.01 was deem to be statistically significant.
The FC between two groups were compared using the two-sample t-test. P<0.01 was considered statistically significant by using Gaussian Random Field (GRF) theory to correct multiple comparisons.
We used receiver operating characteristic (ROC) curve analysis to distinguish the average FC values of different brain regions in patients with RVO and HCs. The relationship between the average FC values in different cerebrum areas and the behavioral results in RVOs were analyzed with Pearson’s correlation analysis. P<0.01 was regarded as statistical threshold.
Results
Demographics and behavioral results
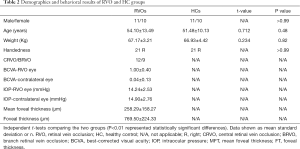
There were no markedly differences in age (P=0.48) and weight (P=0.82) between the RVO patients and the HCs. The best-corrected visual acuity (BCVA) of the RVO eye was 1.00±0.40 logMAR, and the contralateral eye was 0.04±0.13 log MAR (more details were shown in Table 2).
Full table
FC differences
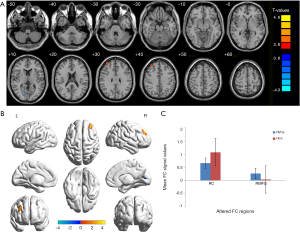
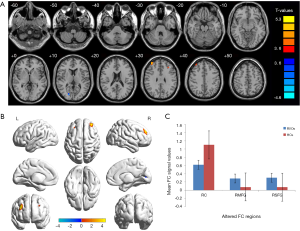
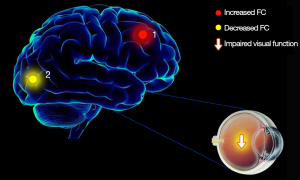
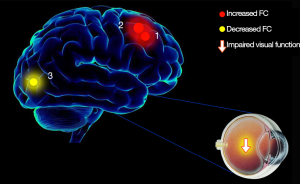
The RVO group exhibited increased FC between the left V1 and the right middle frontal gyrus (BA 9) in comparison with HCs, but decreased FC between the left V1 and right cuneus (BA 17) (Figure 2, Table 3). In the meantime, subjects with RVO showed higher FC between the right V1 and right middle frontal gyrus (BA 9), right superior frontal gyrus (BA 9), but lower FC between right V1 and right cuneus (BA 17) (Figure 3, Table 3).

Full table
Correlation analysis
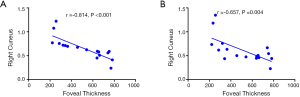
In RVO group, the foveal thickness showed a negative correlation with the FC values between the left V1 and the right cuneus (r=−0.81, P<0.001), and the FC values between the right V1 and the right cuneus (r=−0.66, P=0.004) (Figure 4).
Receiver operating characteristic curve
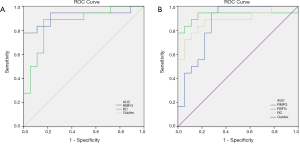
We speculated that the distinction of FC values might be feasible diagnostic biomarkers to classify RVO from HCs. In order to affirm this hypothesis, we used ROC curve method to obtain and analysis average FC values of different cerebrum regions. The individual area under the curve (AUC) of FC values were as follow: right middle frontal gyrus (0.92, P<0.001), right cuneus (0.86, P<0.001) (Figure 5A, left V1); right middle frontal gyrus (0.86, P<0.001), right superior frontal gyrus (0.93, P<0.001), right cuneus (0.89, P<0.001) (Figure 5B, right V1).
Discussion
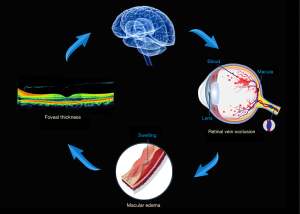
RVO is a general threatening retinal vascular disease which attracts much attention on their underlying pathogenesis. In RVO, retinal vein obstruction leads to intraretinal haemorrhages, retinal venous congestion and macular edema (5), further result in alterations in foveal thickness and even the cerebrum activity (Figure 6).
Seed-based FC is a novel and reliable rs-fMRI method to reveal the alterations of cerebral functional connectivity, exploring regions of activation in the brain. Previous studies have been applied in some ophthalmological diseases (Table 4) and revealed abnormal spontaneous FC between the V1 and the other regions in patients with glaucoma (16), amblyopia (17) and strabismus (18). To our best knowledge, the present study is initial to explore the cerebral FC involved in RVO individuals.
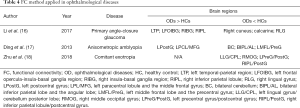
Full table
In this study, we demonstrated that the spontaneous brain functional connectivity between V1 and other different areas in subjects with RVO were altered when compared with HCs. The RVO group exhibited significant increased FC between the left V1 and the right middle frontal gyrus, and lower FC between the left V1 and the right cuneus with impaired visual function (Figure 7). Meanwhile, RVO group showed notably higher FC between the right V1 and right middle frontal gyrus, right superior frontal gyrus, and decreased FC between right V1 and right cuneus with impaired visual function (Figure 8).
Analysis of increased FC values in RVO group
The middle frontal gyrus, constitutes approximately one-third of the frontal lobe of the human brain, lies between the inferior and superior frontal sulci, in front of the precentral gyrus (23). The frontal eye field (FEF) is located around the intersection of middle frontal gyrus with the precentral gyrus, which involves in eye movement and visual attention (24). Converging evidence of functional neuroimaging studies have consistently revealed that the middle frontal gyrus is associated with saccade and movement generation (25,26). Previous study has revealed that FC value was increased in the primary open-angle glaucoma between the V1 and middle frontal gyrus (22). Moreover, researches on several retina-related diseases have observed increased activation in FEF, including retinal detachment (27), age-related macular degeneration (28,29), progressive retinitis pigmentosa (30) and macular hole (31). In line with these preceding results, the increased FC values between bilateral V1 and right middle frontal gyrus shown in this study reflected an activation of the visual processing, which may suggest the plasticity that compensates for RVO-related visual input deficits.
The superior frontal gyrus lies on the superior part of the prefrontal cortex and is bounded laterally by the superior frontal sulcus (32). It’s extensively considered to take a part in the interplay between cognitive related processing (33,34) and motor controlling (35,36). Previous studies about ocular diseases have shown inconsistency in this region. Researches on patients with strabismus have shown that the cerebral blood flow were markedly increased in the superior frontal gyrus (37,38). A similar higher regional homogeneity value was found in acute eye pain individuals when comparing with HCs (39). However, there were studies demonstrated that optic neuritis patients exhibited decreased brain activities in the superior frontal gyrus (40,41). What’s more, Li et al. (42) and Chen et al. (43) both revealed that reduced neural activities of superior frontal gyrus were identified in patients with primary open-angle glaucoma. We inspected that subjects with RVO showed significant higher FC values between right V1 and right superior frontal gyrus, indicating functional injured in this region. Therefore, we further inferred that this might result in the visual impairment in RVO subjects.
Analysis of decreased DC values in RVO group
The cuneus is located in the occipital lobe and bounded by the parieto-occipital sulcus and calcarine sulcus, which is most known for its involvement in visual information processing. It has been reported that the cuneus activates almost simultaneously with the V1 in response to a visual stimulus and may act to modulate signals travelling from the V1 to the extrastriate cortices (44). Several previous functional studies revealed that the cuneus was related to ophthalmological diseases. Investigators illustrated that the functional connectivity of cuneus decreased in on glaucoma patients when comparing with normal subjects (16,45). And reduced gray matter volume of cuneus was observed in strabismic patients (46). Huang et al. (27) investigated that significant decline of brain neural homogeneity was found in cuneus in individuals with retinal detachment, and similar decrease trend in cuneus was reported in acute open globe injury (47). Besides, monocular blindness subjects exhibited lower voxel-mirrored homotopic connectivity values in cuneus. Consistence with these previous findings, we also observed significant FC reduction between bilateral V1 and right cuneus in the current study, as well as the negative correlation with foveal thickness, which indicated the severity of RVO and visual quality. All above might imply functional deficient in RVO patients, which provided new evidence that the RVO could result in dysfunction of the cuneus.
The ROC curve analysis could provide an effectively statistical method to distinguish disease from HCs with high sensitivity and specificity (Figure 5). The accuracy is considered excellent for AUC value is over 0.8, the discrimination results between 0.6 and 0.8 denotes the accuracy is moderate, and if the value less than 0.6, the accuracy is unreliable. In this study, ROC curve analysis found that excellent AUC values were observed among all areas of interest included the right middle frontal gyrus, superior frontal gyrus and cuneus, indicating that FC method is useful for characterizing the neural mechanisms underlying RVO and may be capable of detecting early biomarkers of RVO from HCs.
Nevertheless, some limitations in this study need to be considered. Firstly, a relatively insufficient number of participants were recruited, which may have affected the reliability. In addition, various types of RVO were implicated, which are supposed to be categorized. However, we have not found significant differences between CRVO and BRVO, which might due to the small sample, Subsequent study is needed to probe the functional alterations of the brain more precisely.
Conclusions
In summary, this study found that RVO patients have abnormal intrinsic activity in different brain regions, which might be helpful to understand the neurological variation of RVO patients and had a chance to reveal the potential mechanism of RVO. The FC signals could be an effective biomarker to identify RVO.
Acknowledgments
Funding: This research is supported by National Natural Science Foundation of China (No: 81660158, 81400372, 81460092); Natural Science research Foundation of Guangdong Province (No: 2017A030313614, 2017A020215187, 2018A030313117); Medical Science Foundation of Guangdong Province (No: A2016184).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/qims.2020.03.24). The authors have no conflicts of interest to declare.
Ethical Statement: All research methods were approved by the Medical Ethics Committee of the First Affiliated Hospital of Nanchang University (CDYFY-LL-2017025) and complied with the Helsinki Declaration. The whole study was carried out in accordance with the approved guidelines. All participants were notified the objectives, contents, latent risks, and signed informed consent agreements for this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sivaprasad S, Amoaku WM, Hykin P. The Royal College of Ophthalmologists Guidelines on retinal vein occlusions: executive summary. Eye (Lond) 2015;29:1633-8. [Crossref] [PubMed]
- Yasuda M, Kiyohara Y, Arakawa S, Hata Y, Yonemoto K, Doi Y, Iida M, Ishibashi T. Prevalence and systemic risk factors for retinal vein occlusion in a general Japanese population: the Hisayama study. Invest Ophthalmol Vis Sci 2010;51:3205-9. [Crossref] [PubMed]
- Cugati S, Wang JJ, Rochtchina E, Mitchell P. Ten-year incidence of retinal vein occlusion in an older population: the Blue Mountains Eye Study. Arch Ophthalmol 2006;124:726-32. [Crossref] [PubMed]
- Klein R, Moss SE, Meuer SM, Klein BE. The 15-year cumulative incidence of retinal vein occlusion: the Beaver Dam Eye Study. Arch Ophthalmol 2008;126:513-8. [Crossref] [PubMed]
- McAllister IL. Central retinal vein occlusion: a review. Clin Exp Ophthalmol 2012;40:48-58. [Crossref] [PubMed]
- Jaulim A, Ahmed B, Khanam T, Chatziralli IP. Branch retinal vein occlusion: epidemiology, pathogenesis, risk factors, clinical features, diagnosis, and complications. An update of the literature. Retina 2013;33:901-10. [Crossref] [PubMed]
- Chan CK, Ip MS, Vanveldhuisen PC, Oden NL, Scott IU, Tolentino MJ, Blodi BA. SCORE Study Investigator Group. SCORE Study report #11: incidences of neovascular events in eyes with retinal vein occlusion. Ophthalmology 2011;118:1364-72. [PubMed]
- Chuang LH, Wang NK, Chen YP, Yeung L, Hwang YS, Chen KJ, Wu WC, Chen TL, Lai CC. Vitrectomy and panretinal photocoagulation reduces the occurrence of neovascular glaucoma in central retinal vein occlusion with vitreous hemorrhage. Retina 2013;33:798-802. [Crossref] [PubMed]
- Ip M, Hendrick A. Retinal Vein Occlusion Review. Asia Pac J Ophthalmol (Phila) 2018;7:40-5. [PubMed]
- Kida T. Mystery of Retinal Vein Occlusion: Vasoactivity of the Vein and Possible Involvement of Endothelin-1. Biomed Res Int 2017;2017:4816527. [Crossref] [PubMed]
- Jonas JB, Mones J, Glacet-Bernard A, Coscas G. Retinal Vein Occlusions. Dev Ophthalmol 2017;58:139-67. [Crossref] [PubMed]
- Wen SM, Min YL, Yuan Q, Li B, Lin Q, Zhu PW, Shi WQ, Shu YQ, Shao Y, Zhou Q. Altered spontaneous brain activity in retinal vein occlusion as determined by regional homogeneity: a resting-state fMRI study. Acta Radiol 2019;60:1695-702. [Crossref] [PubMed]
- Wu YY, Yuan Q, Li B, Lin Q, Zhu PW, Min YL, Shi WQ, Shu YQ, Zhou Q, Shao Y. Altered spontaneous brain activity patterns in patients with retinal vein occlusion indicated by the amplitude of low-frequency fluctuation: A functional magnetic resonance imaging study. Exp Ther Med 2019;18:2063-71. [PubMed]
- Lang S, Duncan N, Northoff G. Resting-State Functional Magnetic Resonance Imaging: Review of Neurosurgical Applications. Neurosurgery 2014;74:453-64. [Crossref] [PubMed]
- Armstrong CC, Moody TD, Feusner JD, McCracken JT, Chang S, Levitt JG, Piacentini JC, O'Neill J. Graph-theoretical analysis of resting-state fMRI in pediatric obsessive–compulsive disorder. J Affect Disord 2016;193:175-84. [Crossref] [PubMed]
- Li S, Li P, Gong H, Jiang F, Liu D, Cai F, Pei C, Zhou F, Zeng X. Intrinsic Functional Connectivity Alterations of the Primary Visual Cortex in Primary Angle-Closure Glaucoma Patients before and after Surgery: A Resting-State fMRI Study. PLoS One 2017;12:e0170598. [Crossref] [PubMed]
- Ding K, Liu Y, Yan X, Lin X, Jiang T. Altered functional connectivity of the primary visual cortex in subjects with amblyopia. Neural Plast 2013;2013:612086. [Crossref] [PubMed]
- Zhu PW, Huang X, Ye L, Jiang N, Zhong YL, Yuan Q, Zhou FQ, Shao Y. Altered intrinsic functional connectivity of the primary visual cortex in youth patients with comitant exotropia: a resting state fMRI study. Int J Ophthalmol 2018;11:668-73. [PubMed]
- Yan X, Wang Y, Xu L, Liu Y, Song S, Ding K, Zhou Y, Jiang T, Lin X. Altered Functional Connectivity of the Primary Visual Cortex in Adult Comitant Strabismus: A Resting-State Functional MRI Study. Curr Eye Res 2019;44:316-23. [Crossref] [PubMed]
- Swienton DJ, Thomas AG. The visual pathway--functional anatomy and pathology. Semin Ultrasound CT MR 2014;35:487-503. [Crossref] [PubMed]
- Mock VL, Luke KL, Hembrook-Short JR, Briggs F. Dynamic communication of attention signals between the LGN and V1. Journal of neurophysiology 2018;120:1625-39. [Crossref] [PubMed]
- Dai H, Morelli JN, Ai F, Yin D, Hu C, Xu D, Li Y. Resting-state functional MRI: functional connectivity analysis of the visual cortex in primary open-angle glaucoma patients. Hum Brain Mapp 2013;34:2455-63. [Crossref] [PubMed]
- Petrides M, Pandya DN. Chapter 26 - The Frontal Cortex. In: Mai JK, Paxinos G, editors. The Human Nervous System (Third Edition). San Diego: Academic Press; 2012. p. 988-1011.
- Vernet M, Quentin R, Chanes L, Mitsumasu A, Valero-Cabre A. Frontal eye field, where art thou? Anatomy, function, and non-invasive manipulation of frontal regions involved in eye movements and associated cognitive operations. Front Integr Neurosci 2014;8:66. [PubMed]
- Fernandes HL, Stevenson IH, Phillips AN, Segraves MA, Kording KP. Saliency and saccade encoding in the frontal eye field during natural scene search. Cereb Cortex 2014;24:3232-45. [Crossref] [PubMed]
- Brown MR, Vilis T, Everling S. Isolation of saccade inhibition processes: rapid event-related fMRI of saccades and nogo trials. Neuroimage 2008;39:793-804. [Crossref] [PubMed]
- Huang X, Li D, Li HJ, Zhong YL, Freeberg S, Bao J, Zeng XJ, Shao Y. Abnormal regional spontaneous neural activity in visual pathway in retinal detachment patients: a resting-state functional MRI study. Neuropsychiatr Dis Treat 2017;13:2849-54. [Crossref] [PubMed]
- Little DM, Thulborn KR, Szlyk JP. An FMRI study of saccadic and smooth-pursuit eye movement control in patients with age-related macular degeneration. Invest Ophthalmol Vis Sci 2008;49:1728-35. [Crossref] [PubMed]
- Szlyk JP, Little DM. An FMRI study of word-level recognition and processing in patients with age-related macular degeneration. Invest Ophthalmol Vis Sci 2009;50:4487-95. [Crossref] [PubMed]
- Yoshida M, Origuchi M, Urayama S, Takatsuki A, Kan S, Aso T, Shiose T, Sawamoto N, Miyauchi S, Fukuyama H, Seiyama A. fMRI evidence of improved visual function in patients with progressive retinitis pigmentosa by eye-movement training. Neuroimage Clin 2014;5:161-8. [Crossref] [PubMed]
- Hamamatsu T, Nakagawa Y, Tamai M, Ito M. Visual processing in patients with macular hole. Tohoku J Exp Med 2000;190:249-60. [Crossref] [PubMed]
- Li W, Qin W, Liu H, Fan L, Wang J, Jiang T, Yu C. Subregions of the human superior frontal gyrus and their connections. Neuroimage 2013;78:46-58. [Crossref] [PubMed]
- Owen AM. The role of the lateral frontal cortex in mnemonic processing: the contribution of functional neuroimaging. Exp Brain Res 2000;133:33-43. [Crossref] [PubMed]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci U S A 2006;103:10046-51. [Crossref] [PubMed]
- Martino J, Gabarros A, Deus J, Juncadella M, Acebes JJ, Torres A, Pujol J. Intrasurgical mapping of complex motor function in the superior frontal gyrus. Neuroscience 2011;179:131-42. [Crossref] [PubMed]
- Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci 2008;9:856-69. [Crossref] [PubMed]
- Min YL, Su T, Shu YQ, Liu WF, Chen LL, Shi WQ, Jiang N, Zhu PW, Yuan Q, Xu XW, Ye L, Shao Y. Altered spontaneous brain activity patterns in strabismus with amblyopia patients using amplitude of low-frequency fluctuation: a resting-state fMRI study. Neuropsychiatr Dis Treat 2018;14:2351-9. [Crossref] [PubMed]
- Huang X, Zhou S, Su T, Ye L, Zhu PW, Shi WQ, Min YL, Yuan Q, Yang QC, Zhou FQ, Shao Y. Resting cerebral blood flow alterations specific to the comitant exophoria patients revealed by arterial spin labeling perfusion magnetic resonance imaging. Microvasc Res 2018;120:67-73. [Crossref] [PubMed]
- Tang LY, Li HJ, Huang X, Bao J, Sethi Z, Ye L, Yuan Q, Zhu PW, Jiang N, Gao GP, Shao Y. Assessment of synchronous neural activities revealed by regional homogeneity in individuals with acute eye pain: a resting-state functional magnetic resonance imaging study. J Pain Res 2018;11:843-50. [Crossref] [PubMed]
- Shao Y, Cai FQ, Zhong YL, Huang X, Zhang Y, Hu PH, Pei CG, Zhou FQ, Zeng XJ. Altered intrinsic regional spontaneous brain activity in patients with optic neuritis: a resting-state functional magnetic resonance imaging study. Neuropsychiatr Dis Treat 2015;11:3065-73. [Crossref] [PubMed]
- Huang X, Zhang Q, Hu PH, Zhong YL, Zhang Y, Wei R, Xu TT, Shao Y. Oculopathy fMRI study group. White and Gray Matter Volume Changes and Correlation with Visual Evoked Potential in Patients with Optic Neuritis: A Voxel-Based Morphometry Study. Med Sci Monit 2016;22:1115-23. [Crossref] [PubMed]
- Li T, Liu Z, Li J, Liu Z, Tang Z, Xie X, Yang D, Wang N, Tian J, Xian J. Altered amplitude of low-frequency fluctuation in primary open-angle glaucoma: a resting-state FMRI study. Invest Ophthalmol Vis Sci 2014;56:322-9. [Crossref] [PubMed]
- Chen WW, Wang N, Cai S, Fang Z, Yu M, Wu Q, Tang L, Guo B, Feng Y, Jonas JB, Chen X, Liu X, Gong Q. Structural brain abnormalities in patients with primary open-angle glaucoma: a study with 3T MR imaging. Invest Ophthalmol Vis Sci 2013;54:545-54. [Crossref] [PubMed]
- Vanni S, Tanskanen T, Seppä M, Uutela K, Hari R. Coinciding early activation of the human primary visual cortex and anteromedial cuneus. Proc Natl Acad Sci U S A 2001;98:2776-80. [Crossref] [PubMed]
- Peng Zhou, Jieqiong Wang, Ting Li, Ningli Wang, Junfang Xian, Huiguang He. Abnormal interhemispheric resting-state functional connectivity in primary open-angle glaucoma. Conf Proc IEEE Eng Med Biol Soc 2016;2016:4055-8. [PubMed]
- Chan ST, Tang KW, Lam KC, Chan LK, Mendola JD, Kwong KK. Neuroanatomy of adult strabismus: a voxel-based morphometric analysis of magnetic resonance structural scans. NeuroImage 2004;22:986-94. [Crossref] [PubMed]
- Ye L, Wei R, Huang X, Shi WQ, Yang QC, Yuan Q, Zhu PW, Jiang N, Li B, Zhou Q, Zhou FQ, Shao Y. Reduction in interhemispheric functional connectivity in the dorsal visual pathway in unilateral acute open globe injury patients: a resting-state fMRI study. Int J Ophthalmol 2018;11:1056-60. [PubMed]


