
Spontaneous interventricular septum dissecting hematoma with endocardial fibroelastosis: imaging, diagnosis, surgical therapy and 6-year follow-up outcomes
Introduction
Interventricular septum dissecting hematoma is a rare and fatal cardiac complication with various causes including myocardial infarction, surgery, interventional therapy and trauma (1-3). Although several cases of this condition have been reported among infants and children with congenital heart disease surgery (mainly for ventricular septal defect) (2,4,5), there is no report of spontaneous interventricular septum dissecting hematoma combined with endocardial fibroelastosis. Furthermore, controversy surrounds the treatment for interventricular septum dissecting hematoma. This study reports a rare case of spontaneous interventricular septum dissecting hematoma combined with development of endocardial fibroelastosis. This case highlights imaging appearances of echocardiography and cardiac magnetic resonance (MR) imaging and presents correlated surgical treatment. The case is further supported by a 6-year follow-up study with reports of echocardiographic imaging on a comparative basis.
Case study details
A 20-year-old male patient was admitted to our hospital with onset of sudden chest distress for 24 hours accompanied by progressive dyspnea but no chest pain or fever. Family history showed no evidence of cardiovascular disease. All laboratory blood test results were negative. The electrocardiogram showed ST-segment decline with biphasic or inverted T-wave in II, III, avF, V4 through V6.
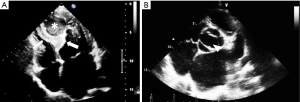
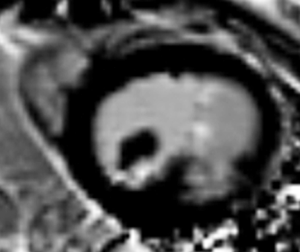
Transthoracic echocardiography (TTE) showed enlargement of both left ventricle and left atrium. Interventricular septum fusiform showed thickening of the middle and apical segments with hypokinesis. Further observations revealed a 34 mm × 36 mm cystic lesion with mixed echo appearance at the septum with 19 mm × 16 mm thrombosis (Figure 1A). The lesion protruded into the left ventricular cavity without obvious obstruction. Endocardial fibroelastosis was indicated by a thickening of the left ventricular endocardium with hyperecho (Figure 1A). Examination also revealed a bicuspid aortic valve (Figure 1B). Systolic pulmonary artery pressure was 66 mmHg. Left ventricular ejection fraction (EF) was 58%.
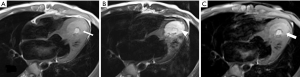
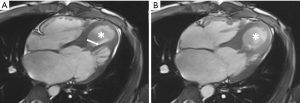
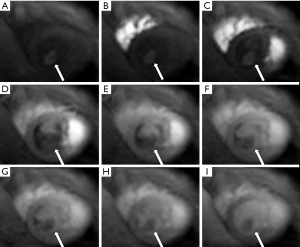
Cardiac MR examination demonstrated hyper-signal intensity of the septal cystic lesion on both T1WI and T2WI images without any signal decrease on fat-suppression sequence (Figure 2). The motion function of interventricular septum was impaired and the lesion could be compressed during the diastolic phase (Figure 3). Perfusion sequence imaging exhibited a heterogeneous enhancement pattern following myocardium enhancement (Figure 4). Myocardium perfusion defect and delay enhancement were both absent during cardiac MR examination (Figures 4 and 5).
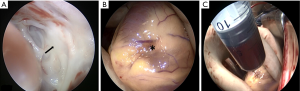
Surgery was performed on the patient. During the procedure endocardioscopy revealed thickness of the left ventricular endocardium with white color (Figure 6A). A 5 mm surface area indentation was observed at the cardiac apex with a branch passing through the myocardium arising from the left anterior descending artery (LAD) (Figure 6B). Puncture resulted in release of myocardium tension and 10 mL blood was drawn out from the lesion (Figure 6C). The blood supply branch artery was ligatured.
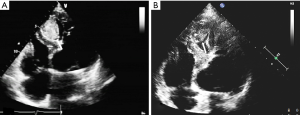
Following surgery, the patient was sent to intensive care unit (ICU) for 24 hours and discharged from hospital one week later. No adverse events occurred during the period of hospitalization. Post-surgery ultrasound examination was conducted after 3 months and repeated after 6 years showing thrombosis formation and decrease in size of the lesion (Figure 7). The patient remained free from discomfort and did not experience any cardiovascular adverse events during the six years following surgery.
Discussion
The present study reports a rare and complex occurrence of spontaneous dissecting hematoma at the interventricular septum in combination with endocardial fibroelastosis. Such a study has not been described earlier. The 20-year-old male subject suffered from bicuspid aortic valve and endocardial fibroelastosis. A branch arising from LAD and passing through the myocardium was observed at the cardiac apex. Cardiac MR examination did not indicate perfusion defect or delay enhancement associated with the lesion.
TTE is the most convenient imaging modality for evaluating the movement and morphology of the ventricular septum. This technique very clearly facilitates assessment of several parameters including interventricular septum hypokinesis/akinesis, thickened septum with mixed or hypoechoic cystic lesion and outflow obstruction, as is evident in the present study. Examination of the lesion by cardiac MR showed high T1WI signal because of filling with blood. Injection of contrast medium enabled enhancement of the interventricular septum during coronary angiography or ventriculography. The treatment of interventricular septum dissecting hematoma is fraught with controversy (1). Previous reports cite a higher incidence of mortality associated with operative therapy in comparison to conservative management (1,5) which may be ascribed to differences in clinical conditions. While patients with stable hemodynamic status usually receive conservative management, emergency surgery represents the more frequently chosen option for those with outflow tract obstruction, severe heart failure and fatal arrhythmia. In the present study, septal dissecting hematoma was drawn out to effectively reduce myocardium pressure and the blood supply branch was subsequently ligatured. During the follow up period, formation of thrombosis and thinning of the interventricular septum were noticed.
This article provides a detailed analysis of imaging findings based on both TTE as well as cardiac MR imaging. In addition, results of surgical intervention are detailed and supported by a follow-up echocardiography examination 3 months and 6 years after surgery in order to confirm the success of treatment outcomes with complete absence of cardiac adverse events. It follows that echocardiography is the preferred imaging modality for timely diagnosis of patients with suspected interventricular septal hematoma.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/qims.2020.03.03). ZS serves as an unpaid editorial board member of Quantitative Imaging in Medicine and Surgery. The other authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vargas-Barrón J, Roldán FJ, Romero-Cárdenas A, Molina-Carrión M, Vázquez-Antona CA, Zabalgoitia M, Martínez Rios MA, Pérez JE. Dissecting intramyocardial hematoma: clinical presentation, pathophysiology, outcomes and delineation by echocardiography. Echocardiography 2009;26:254-61. [Crossref] [PubMed]
- Zhu J, Liu H, Zhang J, Feng X, Wu S, Mei J, Ding F. Interventricular septal hematoma after congenital cardiac surgery. Ann Thorac Surg 2013;95:2171-3. [Crossref] [PubMed]
- Wrzosek J, Plazak W, Dabrowski W, Konieczynska M, Podolec P. Interventricular septum haematoma with right ventricle obstruction caused by percutaneous coronary intervention: case report. Eur J Echocardiogr 2011;12:554. [PubMed]
- Eyileten Z, Aliyev A, Çiftçi Ö, Uçar T, Ödek Ç, Kendirli T, Tutar E, Atalay S, Uysalel A. An extremely rare complication of congenital heart surgery: interventricular septal hematoma. Turk J Pediatr 2013;55:662-4. [PubMed]
- Jacobs S, Rega F, Vlasselaers D, Gewillig M, Meyns B. Dealing with a septal hematoma after switch operation with ventricular septal defect closure. Heart Surg Forum 2010;13:E263-4. [Crossref] [PubMed]


