
MRI biomarkers of disease progression in multiple sclerosis: old dog, new tricks?
Over more than 35 years, advances in magnetic resonance imaging (MRI) techniques and analysis have fundamentally impacted both diagnostic criteria and treatment algorithms for multiple sclerosis (MS), a potentially disabling neurologic condition that affects nearly 1 million people in the United States alone (1). MS results in multi-focal lesions in the gray and white matter of the central nervous system, histopathologically characterized by varying degrees of inflammation, demyelination, axonal loss, gliosis and remyelination. As the disease progresses, the formation of new lesions becomes less frequent and a “degenerative” phase of diffuse axonal injury and accelerated brain atrophy gradually becomes apparent.
Quantitative MRI-based lesion metrics are now routinely incorporated as primary or secondary endpoints in Phase 2 and 3 clinical trials of disease modifying therapies (DMT) for MS. Similarly, MRI detection of gadolinium-enhancing, new T2 or enlarging T2 MS lesions is an established biomarker of ongoing brain inflammation that commonly drives treatment escalation in clinical practice, even in the absence of discernable relapse. The widespread availability of highly accurate, quantitative lesion metrics will further drive individualized approaches in an increasingly complex therapeutic environment.
Despite these advances, sub-clinical MRI-based biomarkers of disease progression or neurodegeneration remain relatively underdeveloped; and are lacking in clinical practice. At the group level, MRI-detected whole brain atrophy (WBA) associates strongly with disability progression (2-5) and is a common secondary endpoint in MS clinical trials. However, WBA measurement in individual patients is confounded by measurement error and biological fluctuations in brain volume; requires expert neuroimaging analysis skills; and is yet to be validated as a clinical-decision making tool.
When highly effective anti-inflammatory DMT are commenced in relapsing, active MS, new lesion formation is rare and WBA rates may return to the normal range. The effect of these therapies on clinical outcomes in patients with later, progressive forms of the disease is modest, though some gains have been made in recent clinical trials of ocrelizumab (6) and siponimod (7) in primary and secondary progressive MS cohorts respectively. Over the next decade, a new era of neuroprotective and remyelinating therapies is expected to complement the existing armamentarium of anti-inflammatory DMT, with promising early studies of agents such as biotin (8) and clemastine (9), among others. The rapidity with which novel mechanisms of neurodegeneration and molecular obstacles to remyelination are being identified (and translated to Phase 2 clinical trials) has exposed a critical unmet need for robust, in-vivo MRI biomarkers for predicting and monitoring disability progression in MS. Approaches to date (Table 1) have included measures of WBA, regional brain atrophy (4,11-13), ventricular volume change (5,14), cervical spinal cord atrophy (15), gray matter diffusion change (16) and white matter lesion magnetization transfer ratio (MTR) change (17). Novel lesion metrics including change in T1 hypointensity volume within slowly expanding lesions (6) and longitudinal change in structural brain connectivity (18) have also been explored.
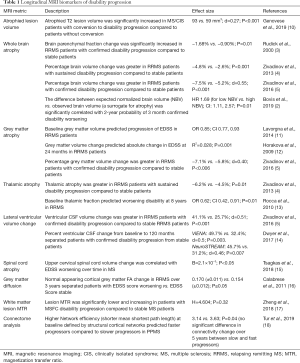
Full table
Recently, Dwyer and colleagues defined a novel lesion metric, “atrophied lesion volume”, representing the volume of lesional T2 hyperintense tissue (periventricular and to a lesser extent gyral) that is subsumed into the cerebrospinal fluid over time by tissue destruction, collapse or both (19). Atrophied lesion volume, which appears to accelerate in later disease, correlated with disability progression over five years in a cohort of patients with clinically isolated syndrome (CIS) and MS. Genovese subsequently investigated this metric in a large cohort of patients with MS (n=1,341) and CIS (n=124) monitored over 4.6 (SD 2.5) years and 3.7 (SD 2.4) years respectively (10). MS patients were dichotomized into those with/without disability progression according to a predefined increase in disability measured by the expanded disability status scale (EDSS); and the association with conventional MRI markers [annualized T2 lesion volume change, WBA and percent ventricular volume change (PVVC)] and atrophied lesion volume was determined. Similarly, patients with relapsing remitting MS (RRMS) and CIS at baseline were classified at last follow up on the basis of conversion to a secondary progressive MS (SPMS) phenotype according to the revised Lublin criteria, and the association between disease course and MRI markers determined. Only 23% of 1,465 patients with MS or CIS exhibited disability progression and 4.6% of this cohort converted to SPMS, noting that some patients were followed for relatively short periods. While patients with disability progression had higher rates of WBA and atrophied lesion volume, there was no association with T2 lesion volume change or, counterintuitively, PVVC. Furthermore, atrophied lesion volume, but neither WBA rate or PVVC, predicted conversion to SPMS. The lack of an association of these conventional MRI metrics with SPMS conversion presumably reflects the sensitivity of atrophied lesion volume as a biomarker of disease progression over relatively short periods of time. The authors propose atrophied lesion volume, a metric that is readily measurable on standard clinically acquired FLAIR and three-dimensional (3D) T1 sequences (20), as a potentially useful tool for routine annual monitoring in clinical practice.
MS is a continuum and the descriptions of CIS, RRMS and SPMS reflect broad clinical observations rather than a fundamental difference in underlying pathophysiology. Accepting this, defining conversion to SPMS on clinical grounds, particularly in individual patients, remains problematic. In particular, the EDSS is a coarse assessment tool that is heavily weighted toward ambulation in later disease, insensitive to changes in cognition confounded by fluctuations related to both patient, treatment and assessor factors. The EDSS is also not universally collected outside major MS centers. The availability of an objective, quantitative MRI biomarker of SPMS conversion would strengthen clinical trials of DMT and, with the availability of therapies that impact disease progression, add clarity to evolving treatment paradigms in clinical practice.
Atrophied lesion volume will require further validation in prospective patient cohorts; in particular, whether routine (annual) application of this metric over the short term will truly predict (rather than associate with) future disability progression is unknown. However, WBA rates over 1–2 years have been shown to predict future disability (21); and the superior sensitivity of atrophied lesion volume as a biomarker of disease progression reported by Genovese et al. suggests that this metric will have true, and potentially greater, predictive power. Atrophied lesion volume inherently requires the presence of periventricular (or visible cortical) pathology on baseline imaging. While there is a clear MS lesion predilection for the periventricular zones, more confluent involvement in this region is usually seen in later disease, potentially compromising the sensitivity of atrophied lesion volume in early RRMS (as a predictor of future disability). Genovese et al. did not stratify their clinical cohort according to DMT. Highly efficacious DMT dramatically reduce the appearance of new and enlarging T2 lesions; and increasing first-line use or rapid treatment escalation to these agents in early RRMS therefore limits the accumulation of periventricular T2 pathology. The utility of atrophied lesion volume, rather than conventional volumetrics (WBA or PVVC) or global lesion/lesion-related change, should therefore be further explored in patient groups in whom modern treatment paradigms have been applied. Finally, atrophied lesion volume may not predict EDSS progression in patients with spinal cord-dominant disease, the principal substrate for the accumulation of motor disability in MS.
The pathological substrates for atrophied lesion volume have not been explored, but tissue destruction and tissue collapse, presumably reflecting severe axon-myelin loss, are the likely principal drivers. Lesion displacement or change in lesion morphology due to ventricular expansion may also be a factor. The authors propose the future application of advanced imaging techniques, such as Jacobian determinant mapping, to determine the relative contribution of true atrophy and tissue collapse/displacement to atrophied lesion volume, and the association of each component with disability progression. By using a binarized lesion mask, atrophied lesion volume is also nescient of the relative destructiveness (degree of axonal loss) of periventricular pathology on baseline imaging. The poor pathological specificity and quantitative precision of conventional T2 imaging contributes to the well described mismatch between disability status and total lesion burden in MS, the so-called clinico-radiological paradox (22). Longitudinal study of microstructure change using diffusion tensor imaging (DTI) demonstrates a progressive increase in isotropic water diffusion within the core of established MS lesions (23), suggesting inflammation-independent, gradual axonal attrition. Together with lesion topography, progressive tissue destruction within lesions and distant effects on connected tracts and synaptically associated neurons (24,25) are the likely principal pathological determinants of clinically apparent progression. Applied globally, a longitudinal measure of axonal loss within lesions might provide a measure of disease progression that is not reliant on the presence of periventricular pathology at baseline and is less likely to be impacted by tissue collapse that may occur at CSF-brain interfaces. Although there are logistical and technical impediments to the application of advanced imaging techniques in both clinical trials and clinical practice, future studies should therefore compare the predictive value of atrophied lesion volume with novel longitudinal global lesion metrics, such change in lesion DTI or MTR.
Another emerging lesion-based metric of disease progression exploits the presence of slowly expanding MS lesions (SELs), recognized on longitudinal T2-weighted scans using a Jacobian based method (26); and analogous with histopathologically identified ‘smouldering’ MS lesions that are common in patients with progressive disease (27). Such lesions are associated with a persistent paramagnetic rim on MRI that correlates with a glial wall comprising microglia, macrophages and proliferating oligodendrocytes; and progressive central T1 hypointensity in the absence of gadolinium enhancement, presumably reflecting significant axonal attrition. In the pivotal Phase 3 trial of ocrelizumab in patients with primary progressive MS (6), an increase in the T1 volume of SELs predicted 12–week composite disability progression (26).
Finally, quantitative analysis of disruption of structural brain connectivity in MS (the ‘disconnectome’) holds promise as a novel MRI biomarker of disease progression. While longitudinal connectivity studies are limited (18), disruption of connectomes on baseline imaging correlates with disease severity (particularly cognition) and may predict future clinical outcomes (18,28). Both traditional network approaches, in which the number of fibres or streamlines (calculated from diffusion MRI) are used to estimate the connectivity strength between brain regions (29); and novel methods that use only a patient white matter lesion mask and a pre-defined healthy control-derived network template, have been investigated in MS cohorts (28). Most recently, Kamagata et al. investigated the brain connectome weighted according to the g-ratio, the ratio of the inner to outer myelinated axon diameter, and reported strong correlation with variation in measures of disease severity in a small cohort (n=14) of patients with MS (30). These findings suggest that demyelination is a substantive driver of brain disconnection in MS, and that the g-ratio weighted disconnectome may be a sensitive biomarker of disease progression.
In summary, there is a veritable smorgasbord of promising lesion (regional and global) and non-lesion based MRI biomarkers of disease progression in various stages of development, clinical validation and integration. Among these, atrophied lesion volume shows promise as a sensitive predictor of disability progression and conversion to SPMS that can be readily measured on clinical quality FLAIR and 3D T1 sequences.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Wallin MT, Culpepper WJ, Campbell JD, Nelson LM, Langer-Gould A, Marrie RA, Cutter GR, Kaye WE, Wagner L, Tremlett H, Buka SL, Dilokthornsakul P, Topol B, Chen LH, LaRocca NG. US Multiple Sclerosis Prevalence Workgroup. The prevalence of MS in the United States: A population-based estimate using health claims data. Neurology 2019;92:e1029-40. [Crossref] [PubMed]
- Bovis F, De Stefano N, Steinerman JR, Knappertz V, Sormani MP. Validating the use of brain volume cutoffs to identify clinically relevant atrophy in RRMS. Mult Scler 2019;25:217-23. [Crossref] [PubMed]
- Rudick RA, Fisher E, Lee JC, Duda JT, Simon J. Brain atrophy in relapsing multiple sclerosis: relationship to relapses, EDSS, and treatment with interferon beta-1a. Mult Scler 2000;6:365-72. [Crossref] [PubMed]
- Zivadinov R, Uher T, Hagemeier J, Vaneckova M, Ramasamy DP, Tyblova M, Bergsland N, Seidl Z, Dwyer MG, Krasensky J, Havrdova E, Horakova D. Evolution of cortical and thalamus atrophy and disability progression in early relapsing-remitting MS during 5 years. AJNR Am J Neuroradiol 2013;34:1931-9. [Crossref] [PubMed]
- Zivadinov R, Uher T, Hagemeier J, Vaneckova M, Ramasamy DP, Tyblova M, Bergsland N, Seidl Z, Dwyer MG, Krasensky J, Havrdova E, Horakova D. A serial 10-year follow-up study of brain atrophy and disability progression in RRMS patients. Mult Scler 2016;22:1709-18. [Crossref] [PubMed]
- Montalban X, Hauser SL, Kappos L, Arnold DL, Bar-Or A, Comi G, de Seze J, Giovannoni G, Hartung HP, Hemmer B, Lublin F, Rammohan KW, Selmaj K, Traboulsee A, Sauter A, Masterman D, Fontoura P, Belachew S, Garren H, Mairon N, Chin P, Wolinsky JS. ORATORIO Clinical Investigators. Ocrelizumab versus Placebo in Primary Progressive Multiple Sclerosis. N Engl J Med 2017;376:209-20. [Crossref] [PubMed]
- Kappos L, Bar-Or A, Cree BAC, Fox RJ, Giovannoni G, Gold R, Vermersch P, Arnold DL, Arnould S, Scherz T, Wolf C, Wallström E, Dahlke F. EXPAND Clinical Investigators. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study. Lancet 2018;391:1263-73. [Crossref] [PubMed]
- Tourbah A, Lebrun-Frenay C, Edan G, Clanet M, Papeix C, Vukusic S, De Sèze J, Debouverie M, Gout O, Clavelou P, Defer G, Laplaud DA, Moreau T, Labauge P, Brochet B, Sedel F, Pelletier J. MS-SPI study group. MD1003 (high-dose biotin) for the treatment of progressive multiple sclerosis: A randomised, double-blind, placebo-controlled study. Mult Scler 2016;22:1719-31. [Crossref] [PubMed]
- Green AJ, Gelfand JM, Cree BA, Bevan C, Boscardin WJ, Mei F, Inman J, Arnow S, Devereux M, Abounasr A, Nobuta H, Zhu A, Friessen M, Gerona R, von Büdingen HC, Henry RG, Hauser SL, Chan JR. Clemastine fumarate as a remyelinating therapy for multiple sclerosis (ReBUILD): a randomised, controlled, double-blind, crossover trial. Lancet 2017;390:2481-9. [Crossref] [PubMed]
- Genovese AV, Hagemeier J, Bergsland N, Jakimovski D, Dwyer MG, Ramasamy DP, Lizarraga AA, Hojnacki D, Kolb C, Weinstock-Guttman B, Zivadinov R. Atrophied Brain T2 Lesion Volume at MRI Is Associated with Disability Progression and Conversion to Secondary Progressive Multiple Sclerosis. Radiology 2019;293:424-33. [Crossref] [PubMed]
- Lavorgna L, Bonavita S, Ippolito D, Lanzillo R, Salemi G, Patti F, Valentino P, Coniglio G, Buccafusca M, Paolicelli D, d'Ambrosio A, Bresciamorra V, Savettieri G, Zappia M, Alfano B, Gallo A, Simone I, Tedeschi G. Clinical and magnetic resonance imaging predictors of disease progression in multiple sclerosis: a nine-year follow-up study. Mult Scler 2014;20:220-6. [Crossref] [PubMed]
- Horakova D, Dwyer MG, Havrdova E, Cox JL, Dolezal O, Bergsland N, Rimes B, Seidl Z, Vaneckova M, Zivadinov R. Gray matter atrophy and disability progression in patients with early relapsing-remitting multiple sclerosis: a 5-year longitudinal study. J Neurol Sci 2009;282:112-9. [Crossref] [PubMed]
- Rocca MA, Mesaros S, Pagani E, Sormani MP, Comi G, Filippi M. Thalamic damage and long-term progression of disability in multiple sclerosis. Radiology 2010;257:463-9. [Crossref] [PubMed]
- Dwyer MG, Silva D, Bergsland N, Horakova D, Ramasamy D, Durfee J, Vaneckova M, Havrdova E, Zivadinov R. Neurological software tool for reliable atrophy measurement (NeuroSTREAM) of the lateral ventricles on clinical-quality T2-FLAIR MRI scans in multiple sclerosis. Neuroimage Clin 2017;15:769-79. [Crossref] [PubMed]
- Tsagkas C, Magon S, Gaetano L, Pezold S, Naegelin Y, Amann M, Stippich C, Cattin P, Wuerfel J, Bieri O, Sprenger T, Kappos L, Parmar K. Spinal cord volume loss: A marker of disease progression in multiple sclerosis. Neurology 2018;91:e349-58. [Crossref] [PubMed]
- Calabrese M, Rinaldi F, Seppi D, Favaretto A, Squarcina L, Mattisi I, Perini P, Bertoldo A, Gallo P. Cortical diffusion-tensor imaging abnormalities in multiple sclerosis: a 3-year longitudinal study. Radiology 2011;261:891-8. [Crossref] [PubMed]
- Zheng Y, Lee JC, Rudick R, Fisher E. Long-Term Magnetization Transfer Ratio Evolution in Multiple Sclerosis White Matter Lesions. J Neuroimaging 2018;28:191-8. [Crossref] [PubMed]
- Tur C, Kanber B, Eshaghi A, Altmann DR, Khaleeli Z, Prados F, Ourselin S, Thompson AJ, Gandini Wheeler-Kingshott CA, Toosy AT, Ciccarelli O. Clinical relevance of cortical network dynamics in early primary progressive MS. Mult Scler 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Dwyer MG, Bergsland N, Ramasamy DP, Jakimovski D, Weinstock-Guttman B, Zivadinov R. Atrophied Brain Lesion Volume: A New Imaging Biomarker in Multiple Sclerosis. J Neuroimaging 2018;28:490-5. [Crossref] [PubMed]
- Zivadinov R, Bergsland N, Dwyer MG. Atrophied brain lesion volume, a magnetic resonance imaging biomarker for monitoring neurodegenerative changes in multiple sclerosis. Quant Imaging Med Surg 2018;8:979-83. [Crossref] [PubMed]
- Popescu V, Agosta F, Hulst HE, Sluimer IC, Knol DL, Sormani MP, Enzinger C, Ropele S, Alonso J, Sastre-Garriga J, Rovira A, Montalban X, Bodini B, Ciccarelli O, Khaleeli Z, Chard DT, Matthews L, Palace J, Giorgio A, De Stefano N, Eisele P, Gass A, Polman CH, Uitdehaag BM, Messina MJ, Comi G, Filippi M, Barkhof F, Vrenken H. MAGNIMS Study Group. Brain atrophy and lesion load predict long term disability in multiple sclerosis. J Neurol Neurosurg Psychiatry 2013;84:1082-91. [Crossref] [PubMed]
- Barkhof F. MRI in multiple sclerosis: correlation with expanded disability status scale (EDSS). Mult Scler 1999;5:283-6. [Crossref] [PubMed]
- Klistorner A, Wang C, Yiannikas C, Parratt J, Dwyer M, Barton J, Graham SL, You Y, Liu S, Barnett MH. Evidence of progressive tissue loss in the core of chronic MS lesions: A longitudinal DTI study. Neuroimage Clin 2017;17:1028-35. [Crossref] [PubMed]
- You Y, Joseph C, Wang C, Gupta V, Liu S, Yiannikas C, Chua BE, Chitranshi N, Shen T, Dheer Y, Invernizzi A, Borotkanics R, Barnett M, Graham SL, Klistorner A. Demyelination precedes axonal loss in the transneuronal spread of human neurodegenerative disease. Brain 2019;142:426-42. [Crossref] [PubMed]
- Klistorner A, Vootakuru N, Wang C, Yiannikas C, Graham SL, Parratt J, Garrick R, Levin N, Masters L, Lagopoulos J, Barnett MH. Decoding diffusivity in multiple sclerosis: analysis of optic radiation lesional and non-lesional white matter. PLoS One 2015;10:e0122114. [Crossref] [PubMed]
- Elliott C, Wolinsky JS, Hauser SL, Kappos L, Barkhof F, Bernasconi C, Wei W, Belachew S, Arnold DL. Slowly expanding/evolving lesions as a magnetic resonance imaging marker of chronic active multiple sclerosis lesions. Mult Scler 2019;25:1915-25. [Crossref] [PubMed]
- Prineas JW, Kwon EE, Cho ES, Sharer LR, Barnett MH, Oleszak EL, Hoffman B, Morgan BP. Immunopathology of secondary-progressive multiple sclerosis. Ann Neurol 2001;50:646-57. [Crossref] [PubMed]
- Kuceyeski A, Monohan E, Morris E, Fujimoto K, Vargas W, Gauthier SA. Baseline biomarkers of connectome disruption and atrophy predict future processing speed in early multiple sclerosis. Neuroimage Clin 2018;19:417-24. [Crossref] [PubMed]
- Shu N, Liu Y, Li K, Duan Y, Wang J, Yu C, Dong H, Ye J, He Y. Diffusion tensor tractography reveals disrupted topological efficiency in white matter structural networks in multiple sclerosis. Cereb Cortex 2011;21:2565-77. [Crossref] [PubMed]
- Kamagata K, Zalesky A, Yokoyama K, Andica C, Hagiwara A, Shimoji K, Kumamaru KK, Takemura MY, Hoshino Y, Kamiya K, Hori M, Pantelis C, Hattori N, Aoki S. MR g-ratio-weighted connectome analysis in patients with multiple sclerosis. Sci Rep 2019;9:13522. [Crossref] [PubMed]


