
One-time high-intensity focused ultrasound ablation of abdominal wall endometriosis with concurrent uterine fibroids or adenomyosis: two cases and literature review
Introduction
Recently, high-intensity focused ultrasound (HIFU) has been widely used in the treatment of uterine fibroids (1,2) and endometriosis (2-4) as a non-invasive regime via a focused ultrasound wave, which increases the tissue temperature through coagulation necrosis. Moreover, HIFU has been proven to be effective and safe in the clinical follow-up of the ablation on abdominal wall endometriosis (AWE) (5-7). However, to date, little is known regarding the treatment of AWE accompanied by uterine fibroids and/or adenomyosis with HIFU.
AWE is a rare type of extra-pelvic endometriosis, which generally shows the obvious characteristics of periodic pain or toughened mass associated with the menstrual cycle (8-12), with increasing incidence due to the rise in the number of cesarean deliveries (8). Fibroids and adenomyosis are the most common benign diseases in women associated with hormone levels during reproductive age. HIFU, with non-bleeding, non-scarring, and quick recovery features, has been the first choice of treatment for AWE in the clinic (5-7).
In this study, we report two patients having AWE combined with uterine neoplasms, who underwent ablation with HIFU. Their baseline characteristics and relevant factors, affecting the process and prognoses, were retrospectively studied, aiming to offer a feasible analysis of treating AWE with concurrent uterine benign diseases, and to further elucidate its potential therapeutic benefits in the clinical setting.
Case presentation
Case 1
A 40-year-old woman [body mass index (BMI): 22.3 kg/m2], presenting with aggravation of dysmenorrhea and increased menstrual flow for 2.5 years, was admitted to hospital on February 15, 2017. She reported menstrual regularity as usual, 6/28 days, with moderate flow (10 sanitary napkins) and dysmenorrhea, and a visual analogue scale (VAS) score of 10 points. Her last menstrual period was on November 14, 2016. Painful bleeding had aggravated 2 years ago, with a VAS score of 10, extended menstrual period for 2 months, and use of up to 30 sanitary napkins during the entire menstrual period. Oral ibuprofen was prescribed for pain relief. Uterine adenomyosis was diagnosed with ultrasound imaging in Beijing Tong Zhou Obstetrics and Gynecology Hospital. Blood transfusion and diagnostic curettage were offered as the menstrual amount was thrice that in the past, causing anemia, and the level of hemoglobin was as low as 50 g/L. Pathology showed no malignant lesions. A levonorgestrel-releasing intrauterine system (LNG-IUS) was offered. However, spotting continued for 3 months, and small vaginal bleedings occurred after every 2–3 months, providing dysmenorrhea relief.
After the LNG-IUS fell off 5 months ago, there was a significant increase in the frequency of menstrual cramps since 4 months, and painful bleeding reappeared. Diagnostic curettage was performed again in a local hospital. The findings of tissue biopsy were partial hyperplasia of endometrial glands, partial secretion changes, and focal interstitial hemorrhage. Although the volume of uterine bleeding was reduced after the administration of leuprorelin (Takeda, Jap.) for about three menstrual cycles, uterine shrinkage was not obvious. She was referred for treatment in our hospital, 11 days ago. Pelvic examination showed that the size of the uterus was similar to that in the 8th week of pregnancy. HIFU ablation was recommended.
Her history showed delivery of a baby boy through cesarean section in 2009, and removal of a right cyst by open abdominal cystectomy in 2013. In 2014, she had undergone transfusion for severe anemia.
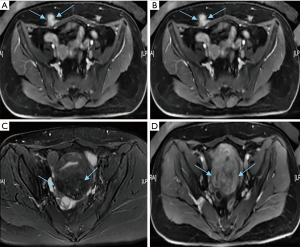
Before HIFU ablation, magnetic resonance imaging (MRI) showed that the uterus was anteriorly located. On T2-weighted MRI, uterine adenomyosis appeared inhomogeneous (hypointensity interspersed with punctiform hyperintensity) on the posterior wall, while contrast-enhanced T1-weighted MRI showed slight inhomogeneous enhancement compared to the myometrium. The posterior myometrium was enlarged by a volume of 137.82 cm3, and AWE in the caesarean scar had a volume of 19.08 cm3 (Figure 1).
Before HIFU, gut preparation, skin degreasing, and degassing were performed. The procedure was the same as previously reported (3,5). Intravenous contrast was administered prior to HIFU. At first, uterine adenomyosis was ablated with HIFU, followed by targeting the AWE.
The sonication and treatment durations were 1,036 s and 92 min, respectively, for the ablation of a 3.83 cm3 lesion, with a mean power of 400 W, total energy of 414,400 J, acquired non-perfusion volume of 86.23 cm3, and non-perfusion volume rate of 62.54% in the uterine posterior wall. Next, sonication and treatment of cutaneous endometriosis took 158 s and 32 min, respectively, for ablation of a 1.43 cm3 lesion, with a mean power of 100 W, total energy of 13,800 J, acquired non-perfusion volume of 4.32 cm3, and non-perfusion volume rate of 77.36% (Figure 2). During the process, the VAS was approximately 7–8 points with intravenous administration of fentanyl (0.8–1 µg/kg) and midazolam hydrochloride (0.02–0.03 mg/kg) for sedation and analgesia. After treatment, the VAS reduced to 5 points, with the pain lasting for 2 h, after which it vanished without any therapy. The intraoperative reaction mainly included pain around the treatment area and sacral burning, and rarely, skin burning.
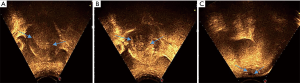
No severe complications occurred during the 18 months of follow-up. At the 1-year follow-up after treatment, the dysmenorrhea score dropped to 2 points, menstrual flow was reduced by half, and the shrinkage rate of the uterine lesion was 63.88%, while the wall of the foci had vanished.
Case 2
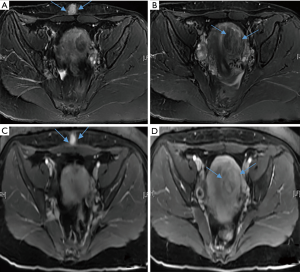
A 41-year-old woman (BMI 24.34 kg/m2), presenting with myomas for 6 years, was admitted to the hospital on February 16, 2017. Her menstrual cycle was regular, at 7/30 days, with light flow and no dysmenorrhea. The last menstrual period was on February 6, 2017. She had undergone removal of hysteroscopic mucosal fibroids, involving intraoperative conversion to open surgery for uterine perforation, 10 years ago. In 2011, laparoscopic resection of the right uterine horn and the fallopian tube was performed after a right-side uterine horn pregnancy. At the time, uterine fibroids of 3.05 cm3 volume were found, but not treated. The follow-up was continued, and the fibroids were found to be increasing in size. In 2016, ultrasound showed multiple fibroids, approximately 143.63 cm3 in volume. Although she delivered a child via cesarean section in 2004, she wanted to conceive another baby. Before treatment, MRI displayed an anterior uterus with a volume of 78.84 cm3, left lateral myoma with a volume 31.82 cm3, and AWE with a volume of 1.92 cm3 in the caesarean scar (Figure 3).
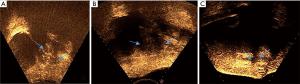
The pretreatment preparation and HIFU process were similar to those described above. The sonication and treatment took 809 s and 98 min, respectively, acquiring an ablation of a 3.98 cm3 lesion, with a mean power of 400 W, total energy of 279,200 J, non-perfusion volume of 22.06 cm3, and non-perfusion volume rate of 62.54% of the uterine lateral fibroids. Then, ablation of the cutaneous endometriosis was performed with sonication for 67 s (100 W–43 s, 400 W–24s, respectively), and treatment for 9 min for a 0.97 cm3 lesion, with a mean power of 207 W, total energy of 13,900 J, non-perfusion volume of 0.50 cm3, and non-perfusion volume rate of 73.86% (Figure 4). During the process, the VAS score was approximately 6 points. The primary intraoperative finding included pain in the treatment area. Otherwise, Case 2 appeared thrice transient conductive fever in the left leg. After the process, pain symptoms disappeared. No severe complications occurred during the 18-month follow-up. The volume of uterine fibroids and AWE reduced by 65% and 73%, respectively, after 1 month. Although the patient was naturally pregnant at 3 months after HIFU, the pregnancy was terminated due to the cessation of fetal development. AWE disappeared after 3 months post-HIFU. The shrinkage rate of ablated fibroids was 31.8% after 1 year.
Discussion
For adenomyosis, it is imperative to draft comprehensive personalized treatment methods for patients of different ages and conditions. One of our patient’s medical history data showed that dysmenorrhea was relieved after conservative treatment with gonadotropin-releasing hormone analog (GnRH-α) and the placement of LNG-IUS, but relapse occurred after termination of medicine and conception. Although the patient had undergone multiple conservative and surgical treatments, symptoms reappeared. Until now, there is still no effective treatment for adenomyosis. The patient had a history of caesarean section and a cyst removal procedure, and AWE was found on MRI.
The prevalence of AWE is rare, estimated to be approximately 0.03–1% and 0.03–3.5% in cases before and after obstetric procedures, respectively (8). The incidence rates of AWE ascend with the increasing trend of cesarean delivery corresponding to the two-baby policy in China (9). The iatrogenic factors related to endometrial debris pollution and dissemination during open surgery are considered to be the etiology of AWE (8). Periodic or toughened painful masses are the most common findings in AWE (10,11). Most patients and physicians believe that AWE need not be tackled if it does not induce uncomfortable symptoms. However, the clinical scenario is somewhat contradictory. In the clinic, many AWE cases show atypical features without cyclic pain or skin protrusions, mostly noted accidentally or on pelvic imaging. Our cases were diagnosed based on only MRI findings. Due to the ability of invasive growth, AWE can expand in different directions and has a detrimental effect on the adjacent tissue. In our hospital, it can be determined promptly using a minimally traumatic method. HIFU ablation was performed on diffuse uterine adenomyosis, as well as extra-pelvic endometriosis as a non-invasive method. The only required condition for thermal treatment is that the lesion ablated should be located in the sonic pathway, which is different from that observed in other modalities.
The analysis of disease history of the patient in Case 2, who desired fertility, showed that removal of the right uterine horn, oviduct, uterine fibroids, left uterine horn, and myometrial fibroids on the left side of the uterine wall had a detrimental effect on embryo implantation. The shrinking of the ablated intramural fibroids transformed the spatial structures, further ameliorating the internal environment in the uterine cavity, which had a significant effect on the fertilized egg implantation. The woman, enduring multiple operations, suffered from tremendous psychological pressure. HIFU offered her a good choice with no scar or bleeding. Meanwhile, it resolved all her lesions at once. A previous study reported that 1 month after HIFU, pregnancy was uneventful. HIFU only ablates the lesion and does not induce any effect on the uterine myometrial structure or function. Conception can be attempted 1 month after the treatment and the woman in the present case had a pregnancy 3 months after undergoing HIFU. Some other studies have yielded similar conclusions (12).
In patients with a history of multiple surgeries, the pelvic organs have different degrees of adhesion with surrounding organs, which has a detrimental effect on treatments, especially on surgery. Thorough cleansing enema, skin degreasing, and degassing before HIFU procedure are crucial in such cases. During the thermal ablation process, close attention should be paid to changes in the skin and intestinal peristalsis around the uterus. A safe distance of 10–15 mm is maintained between the focused sector and the uterine serosa.
It is difficult for other treatments, such as open surgery or laparoscopy, to resolve the lesions existing at the different sites. In contrast, it is easy and safe to use HIFU to excise all lesions located in the treatment path. Minimally invasive modalities, such as laparoscopy and hysteroscopy, must be chosen for fibroids located on the uterine sites. The advantage of HIFU as a non-invasive procedure is not only that it does not cause bleeding, but also its flexibility, especially for outpatient surgery, and reduction of the hospital and patient costs.
Uterine fibroids and/or adenomyosis are the most common benign gynecologic diseases in women during reproductive age, and relapse easily after treatment, associated with hormone levels. Meanwhile, the benign and malignant lesions in the uterus must be differentiated before treatment. HIFU induces cell protein coagulation necrosis in the focal area through a steep temperature increase from 65 to 100 °C in vivo, irrespective of the location and number of lesions, as long as they are in the sonic pathway. The biggest advantage of this procedure is the avoidance of surgery and anesthesia, with no bleeding, and only sedation (2,7). It has been widely used in gynecological diseases, indicating that it is safe and effective (2-7). To date, it has not been reported that HIFU can simultaneously ablate a uterine lesion in combination with AWE.
The thermal ablation can be performed in women with benign gynecological diseases many times without inpatient requirements. For patients with a history of caesarean section, attention should be paid to medical history, physical examination, and imaging checkup (13). For AWE especially located in the muscle layer near the abdominal cavity, the range of which is comparatively large, choosing open surgery as treatment makes it significantly easy to induce abdominal wall defect and insert artificial patch. When uterine diseases coexist with AWE, the choice of surgical methods is more prominent and trickier.
In conclusion, HIFU therapy, as a safe and non-invasive modality, could resolve all lesions presented in the different parts, but located in the sonic pathway, with superior efficacy, while keeping the organ integrity. The shortcoming of this study is that there were only two patients, as these cases are rare. From the experience of two women who underwent ablation with HIFU and their posttreatment follow-up, correlated with the review of the references, we propose that the advantages of HIFU thermal ablation are considerable, compared to other therapies, such as medical intervention or surgery.
Acknowledgments
We are grateful to the women who were willing to provide consent for publishing this study. We also thank our medical team for their help and support.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Duc NM, Keserci B. Emerging clinical applications of high-intensity focused ultrasound. Diagn Interv Radiol 2019;25:398-409. [Crossref] [PubMed]
- Chen J, Chen W, Zhang L, Li K, Peng S, He M, Hu L. Safety of ultrasound-guided ultrasound ablation for uterine fibroids and adenomyosis: A review of 9988 cases. Ultrason Sonochem 2015;27:671-6. [Crossref] [PubMed]
- Feng Y, Hu L, Chen W, Zhang R, Wang X, Chen J. Safety of ultrasound-guided high-intensity focused ultrasound ablation for diffuse adenomyosis: A retrospective cohort study. Ultrason Sonochem 2017;36:139-45. [Crossref] [PubMed]
- Zhang XY, Guo YS, Chen JM, Wang JJ, Ye H, Zang CY, Duan H. Effect of pre-treatment with gonadotropin-releasing hormone analogue GnRH-α on high-intensity focused ultrasound ablation for diffuse adenomyosis: a preliminary study. Int J Hyperthermia 2018;34:1289-97. [Crossref] [PubMed]
- Zhu X, Chen L, Deng X, Xiao S, Ye M, Xue M. A comparison between high-intensity focused ultrasound and surgical treatment for the management of abdominal wall endometriosis. BJOG 2017;124 Suppl 3:53-8. [Crossref] [PubMed]
- Xiaoying ZH., Hua D. Effect of high-intensity focused ultrasound ablation on endometriosis of the abdominal wall. Int J Clin Exp Pathol 2018;11:2118-24. [PubMed]
- Zhang XY, Duan H, Wang JJ, Guo YS, Cheng JM, Ye H, Zhang CY. Clinical analysis of high-intensity focussed ultrasound ablation for abdominal wall endometriosis: a 4-year experience at a specialty gynecological institution. Int J Hyperthermia 2019;36:87-94. [Crossref] [PubMed]
- Lopez-Soto A, Sanchez-Zapata MI, Martinez-Cendan JP, Ortiz Reina S, Bernal Mañas CM, Remezal Solano M. Cutaneous endometriosis: Presentation of 33 cases and literature review. Eur J Obstet Gynecol Reprod Biol 2018;221:58-63. [Crossref] [PubMed]
- Ding Y, Zhu J. A retrospective review of abdominal wall endometriosis in Shanghai. China. Int J Gynaecol Obstet 2013;121:41-4. [Crossref] [PubMed]
- Marras S, Pluchino N, Petignat P, Wenger JM, Ris F, Buchs NC, Dubuisson J. Abdominal wall endometriosis: An 11-year retrospective observational cohort study. Eur J Obstet Gynecol Reprod Biol X 2019;4:100096. [Crossref] [PubMed]
- Zhang P, Sun Y, Zhang C, Yang Y, Zhang L, Wang N, Xu H. Cesarean scar endometriosis: presentation of 198 cases and literature review. BMC Womens Health 2019;19:14. [Crossref] [PubMed]
- Li JS., Wang Y., Chen JY., Chen WZ. Pregnancy outcomes in nulliparous women after ultrasound ablation of uterine fibroids: A single-central retrospective study. Sci Rep 2017;7:3977. [Crossref] [PubMed]
- Koninckx PR, Ussia A, Wattiez A, Zupi E, Gomel V. Risk factors, clinical presentation, and outcomes for abdominal wall endometriosis. J Minim Invasive Gynecol 2018;25:342-3. [Crossref] [PubMed]


