
Chest computed tomography semi-quantitative pleural effusion and pulmonary consolidation are early predictors of acute pancreatitis severity
Introduction
Acute pancreatitis (AP) is an inflammatory disorder that leads to a wide range of local and systemic pathophysiological changes, and can have myriad clinical manifestations and prognoses (1). AP is a dynamic process with 2 overlapping phases of the disease: the early phase, which lasts 1 week, and the following late phase, which lasts for weeks to months (2). In the early phase, the severity of AP primarily depends on the presence and duration of organ failure due to a systemic inflammatory response (2), but is not correlated with the infection or the extent of necrosis. Thus, the prediction of severe AP and organ failure in the early phase is particularly important to evaluate the prognoses of AP.
AP can cause extensive local and systemic pathophysiological changes. Thoracic complications in AP patients include pleural effusion, pulmonary consolidation, atelectasis, pericardial effusion, elevated diaphragms, mediastinal pseudocysts, and pulmonary embolism (3-6). Of these, pleural effusion and pulmonary consolidation are common and are important causes of morbidity, posing diagnostic and therapeutic challenges (3). In 22–29% of the deaths of patients with AP, intrathoracic complications are the major factor, while they are the contributing factor in a further 29–39% of deaths (3).
Computed tomography (CT) is commonly used to diagnose AP and determine the extent of AP with high accuracy and sensitivity (6). CT scans are fast and have a high spatial resolution (7). CT severity index (CTSI) can assess the severity of AP, especially local complications (4-6). However, the use of contrast agents may induce significant alterations in the microcirculation of the pancreas and adversely affect the course of AP (8). The acute physiology and chronic health evaluation II (APACHE II) scoring system can determine the systemic complications of AP (9). The bedside index for severity in acute pancreatitis (BISAP) score is a simple and accurate method for the early identification of patients at increased risk for in-hospital mortality and morbidity (10). However, APACHE II and BISAP scores are difficult to calculate because a great deal of baseline data needs to be collected.
A chest radiograph is the primary imaging modality for evaluating pleural effusion and pulmonary consolidation in the setting of pancreatitis. Typically however, the radiographs are taken at the bedside (portable), and due to the inadequate positioning and supine nature, mild-to-moderate effusions may be missed. Due to image overlap, a subtle pulmonary consolidation may be ignored. Ultrasound has some limitations in the diagnosis of lung consolidation because of gas interference. Currently, CT volumetry is considered the gold standard for the accurate volume measurement of pleural effusion in patients (11). CT is helpful for the accurate evaluation of pleural effusion and pulmonary consolidation in AP patients. However, to our knowledge, most of published articles adopted qualitative analysis instead of quantitative analysis (6,12,13). Only Liu et al. (4) measured the thickness of the pleural effusion and pulmonary consolidation on axial CT images in quantitative fashion. However, the scanning range of CT imaging in their study did not include the whole chest. Thus far, there is no report evaluating pleural effusion volumetry by CT in AP patients.
The purpose of this study includes the following: (I) evaluating the prevalence of pleural effusion and pulmonary consolidation in AP based on chest CT images; (II) analyzing the relationship between pleural effusion volume or pulmonary consolidation lobes and the severity of AP based on the CTSI, APACHE II scoring system, and BISAP score; (III) evaluating the utility of the pleural effusion volume or pulmonary consolidation lobes for predicting severe AP and organ failure in the early phase of AP.
Methods
Ethics statement
This study (including contrast-enhanced CT) gained approval from the Institutional Review Board in Panzhihua Central Hospital. All the patients signed informed consent before undergoing CT scans.
Patient population
Consecutive patients with AP in Panzhihua Central Hospital between July 2017 and August 2018 were included in this study. AP was diagnosed using the 2012 Atlanta standard (14). The inclusion criteria for patients were as follows: (I) acute onset, (II) in-patient, (III) first occurrence of AP, (IV) excluding other causes of amylase elevation, and (V) taking thorax-abdominal CT examination within 2 days of onset. A total of 377 AP patients met the inclusion criteria in the study. The exclusion criteria in this study were as follows: (I) pre-existing pleural effusion (n=18), (II) hypoproteinemia (n=7), (III) chronic pancreatitis (n=34), and (IV) tumors or inflammation of intra- or retroperitoneal (n=9). Finally, 309 patients were enrolled in the study. Among them, 196 were male, 113 were female, and the average age was 50±16 years.
Laboratory and clinical data
Baseline data collected included APACHE II and bedside index for severity in AP (BISAP) within 24 hours of admission to the hospital. According to the APACHE II score, the AP patients were classified as mild (0–7 points) or severe (≥8 points) (9). According to the BISAP score, the AP patients were classified as mild (0–2 points) or severe (≥3 points) (10).
The 2012 revised Atlanta standard defined the classification of AP into 3 degrees of severity: mild (absence of organ failure and the absence of local or systemic complications), moderately severe (presence of transient organ failure or local or systemic complications in the absence of persistent organ failure), and severe AP (presence of persistent organ failure) (14). Transient organ failure was defined as organ failure lasting ≤48 hours, and persistent organ failure was defined as organ failure lasting >48 hours. Organ failure can be assessed by respiratory, cardiovascular, and renal systems (14), and based on the modified Marshall scoring system, organ failure was defined as a score of 2 or higher in 1 of the 3 organ systems.
CT imaging technique
All patients underwent CT scans with a double-source scanner (SOMATOM Definition, Siemens Healthcare, Forchheim, Germany). The parameters of chest CT were as follows: 120 kVp, 100 mAs, a detector with a configuration of 64 mm×0.6 mm, pitch size of 1.2, gantry rotation time of 0.5 s, section thickness of 8.0 mm, and a standard reconstruction algorithm. The scans covered the range from the top to the bottom of the lung. The unenhanced chest CT scan was performed on all patients at the same time as abdominal CT.
The parameters of CT for the upper abdomen were 120 kVp, 200 mAs, a detector with a configuration of 64 mm× 0.6 mm, pitch size of 1.2, a gantry rotation time of 0.5 s, section thickness of 5.0mm, and a standard reconstruction algorithm. First, the unenhanced abdomen CT scans were performed, and then contrast material (Iopamiron 300, Schering, Berlin, Germany) was administered at a flow rate of 3–5 mL/s. The scans extended from the diaphragmatic dome to the iliac crest. Of the 309 AP patients, 224 had both an unenhanced abdominal scan and contrast-enhanced scan, while 85 had only an unenhanced abdominal scan. The 85 patients with only an unenhanced abdominal CT scan underwent an additional plain MR scan in the same period or within 72 hours of the CT scanning to identify the pancreatic necrosis.
CT image analysis
The CT image data were delivered to the post-processing station (Syngo MMWP VE 31H, Siemens Healthcare, Forchheim, Germany). The CT image was reviewed by two observers, one with 3 years’ experience in interpreting chest and abdominal CT imaging and the other with 7 years’ experience. Both reviewers were blinded to the clinical outcome and laboratory data.
The volume of pleural effusion was calculated using a semi-automated volumetric software with contour limiting and threshold analysis on an Easyvison workstation (Volume, Syngo MMWP VE 31H, Siemens Healthcare, Forchheim, Germany). A threshold analysis was used to obtain the calculated volume. First, the electronic cursor was used to select the crude outlines of the region in each CT slice, including the pleural fluid and surrounding margin. Then, the Hounsfield value of each voxel in the selected area was measured using system software, and all the voxels with the Hounsfield values in the range of “−50 to 100” were considered as pleural fluid. Each image slice from the CT imaging was analyzed and evaluated individually using the above steps. Next, the total volume of the pleural effusion was calculated using a computer program by summing all the delineated regions and the total slice thickness (Figure 1). The pleural effusion volume was the sum of the bilateral pleural effusion volume.
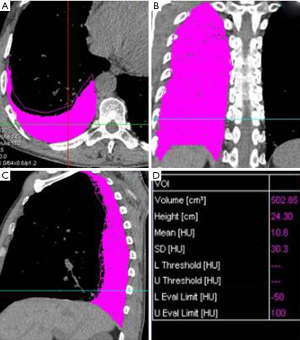
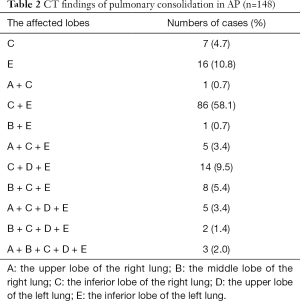
The severity of pulmonary consolidation on CT was graded according to the number of the lobes of the pulmonary consolidation. On CT images, we divided the pulmonary consolidation into 5 lobes: the upper lobe of the right lung, the middle lobe of the right lung, the inferior lobe of the right lung, the upper lobe of the left lung, and the inferior lobe of the left lung. The number of pulmonary consolidations in each lobe was recorded separately. Each lobe with a pulmonary consolidation was assigned 1 point. No pulmonary consolidations were assigned 0 points. The severity of the pulmonary consolidation on CT was scored between 0 and 5 points according to the lobes of the pulmonary consolidation.
The severity of AP was evaluated using the CTSI in the contrast-enhanced CT (5,6). AP was graded as mild (0–3 points), moderate (4–6 points), or severe (7–10 points) based on the CTSI (6). The CTSI for 85 patients who took only an unenhanced abdominal scan was evaluated by combining the plain MR images with the CT images.
Statistical analysis
Results of the pleural effusion volume and pulmonary consolidation scores were given as the mean of the 2 raters. The kappa (k) statistic was used to estimate the inter-rater agreement for the prevalence of pleural effusion and pulmonary consolidation.
Quantitative results were expressed as either the median or the mean ± standard deviation (SD). Continuous variables were compared with Student’s t-tests or Mann-Whitney U tests, Kruskal-Wallis H, and Student-Newman-Keuls test. Categorical variables and rank variables were presented as percentages and compared by the Chi-squared test.
Correlation analyses between pleural effusion volume or pulmonary consolidation scores and different scoring systems, between pleural effusion volume or pulmonary consolidation scores and the length of hospitalization, and between pleural effusion volume or pulmonary consolidation scores and severity of organ failure, were completed with Spearman’s rank correlation tests. A Spearman’s correlation coefficient with an absolute value with a range of 0.090–0.099 was defined as indicating no correlation, those with a range of 0.10–0.29 were defined as a weak correlation, those with a range of 0.30–0.49 were defined a moderate correlation, and those with a range of 0.50–1.0 range were defined as a strong correlation.
Receiver operating characteristic (ROC) analysis was performed to examine the predictive effect of pleural effusion volume, pulmonary consolidation, and scorings for predicting severe AP and organ failure. The discriminative powers of the pleural effusion volume and pulmonary consolidation scores were visualized using ROC curves, including the area under the curve (AUC), with a 95% confidence interval (CI). Additionally, the AUC values of the two parameters were compared using the z-test.
Statistical analysis was performed using commercially available software (SPSS 13.0 version, Chicago, IL, USA), except for the comparison of the AUC of the two scoring systems, which was done with MedCalc 11.6 (MedCalc Software, Mariakerke, Belgium). P values ≤0.05 were considered significant.
Results
Patient characteristics
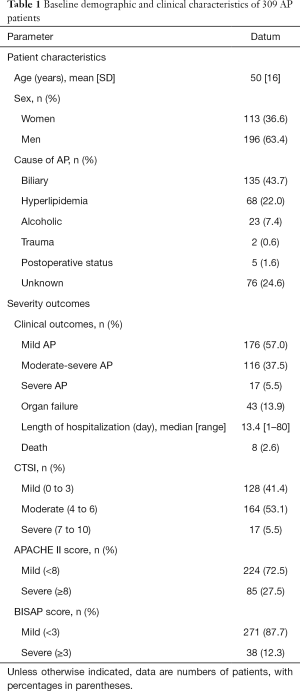
A total of 309 patients were included. Baseline demographic and clinical characteristics are depicted in Table 1. According to the 2012 revised Atlanta standard, 57.0% (176/309) of patients were considered to have mild AP, 37.5% (116/309) of patients were considered to have moderate-severe AP, and 5.5% (17/309) of patients were considered to have severe AP. According to the modified Marshall scoring system, organ failure was present in 43 patients, of whom 26 and 17 patients had transient organ failure and persistent organ failure, respectively. Respiratory failure was observed in 34 patients, of whom 21 and 13 patients had transient and persistent organ failure, respectively. Renal failure was present in 7 patients, of whom 5 and 2 patients had transient and persistent organ failure, respectively. No patients had cardiovascular failure. More than 1 organ system failed in 2 enrolled patients. The mean APACHE II score was 5.8±5.1 points (range, 0–33 points), the mean BISAP score was 1.3±1.0 points (range, 0–5 points), and the mean CTSI was 3.7±1.8 points (range, 0–10 points).
Full table
Pleural effusion and pulmonary consolidation on CT
There was a good agreement between the observers regarding the presence of pleural effusion (κ =0.906, P=0.000) and pulmonary consolidation (κ =0.832, P=0.000) on the CT imaging.
In 309 AP patients, 39.8% (123/309) had pleural effusion (Figures 2-4). Among the 123 patients with pleural effusion, 4.9% (6/123) had right pleural effusion, 30.1% (37/123) had left pleural effusion, and 65.0% (80/123) had bilateral pleural effusion (among the 3 groups, P=0.000; between right and bilateral, P=0.000; between left and bilateral, P=0.000; and between right and left, P=0.000). The mean pleural effusion volume was 41.7±38.0 mL (range, 0–1,079 mL).
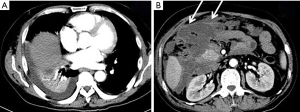
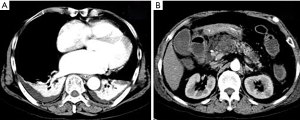
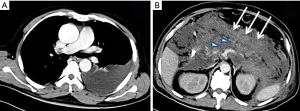
In 309 AP patients, 47.9% (148/309) had pulmonary consolidation (Figures 2,3). The pulmonary consolidation usually occurred in bilateral lower lobes of the lung. The specific distribution details are listed in Table 2. The mean pulmonary consolidation score was 1.0±1.2 points (range, 0–5 points).
Full table
The correlation of pleural effusion and pulmonary consolidation with each scoring system and the day of hospital duration
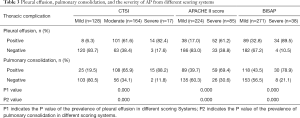
The prevalence of pleural effusion and pulmonary consolidation for each group of different scoring systems is shown in Table 3 (for each group, P=0.000). The pleural effusion volume was strongly correlated with the CTSI score (r=0.574, P=0.000) and BISAP score (r=0.618, P=0.000), but weakly correlated with the APACHE II score (r=0.298, P=0.000) and the length of hospitalization (r=0.249, P=0.000). The pulmonary consolidation score was moderately correlated with the CTSI score (r=0.487, P=0.000), APACHE II score (r=0.348, P=0.000), and BISAP score (r=0.466, P=0.000), but weakly correlated with the length of hospitalization (r=0.216, P=0.000).
Full table
The utility of the pleural effusion volume and pulmonary consolidation scores for predicting the severe AP and organ failure
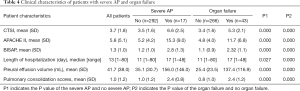
Increased pleural effusion volume and pulmonary consolidation scores were associated with the occurrence of severe AP (P=0.000) and organ failure (P=0.000, Table 4).
Full table
The AUCs of pleural effusion volume for predicting severe AP and organ failure were 0.839 (95% CI: 0.793–0.878) and 0.783 (95% CI: 0.733–0.828), respectively; when the cutoff for severe AP was 52.2 mL or greater, the sensitivity and specificity were 82.35% and 84.93%, respectively; and when the cutoff for organ failure was 47.3 mL or greater, the sensitivity and specificity were 67.44% and 88.35%, respectively. The AUCs of pulmonary consolidation scores for predicting severe AP and organ failure were 0.805 (95% CI: 0.738–0.872) and 0.808 (95% CI: 0.760–0.850), respectively; when the cutoff for severe AP was 2 points or greater, the sensitivity and specificity were 94.12% and 62.67%, respectively; and when the cutoff for organ failure was 2 points or greater, the sensitivity and specificity were 88.37% and 67.29%, respectively (Figure 5).
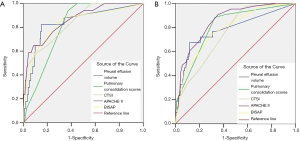
Comparisons of the predictive values of pleural effusion volume and pulmonary consolidation scores and each scoring system for severe AP and organ failure
The CTSI scores, APACHE II scores, BISAP scores, and the length of hospitalization of patients with severe AP were significantly higher than those of patients with mild-to-moderate severe AP (for CTSI scores P=0.000, for APACHE II scores P=0.000, for BISAP scores P=0.000, and for the length of hospitalization P=0.027; Table 4), and the aforementioned indicators were also significantly higher for patients with organ failure than those without organ failure (P=0.000, Table 4).
In predicting severe AP, the accuracy of pleural effusion volume was similar to that of the CTSI scores (P=0.961), APACHE II scores (P=0.757), and BISAP scores (P=0.906; Table 5), and the accuracy of pulmonary consolidation scores was also similar to that of the CTSI scores (P=0.503), APACHE II scores (P=0.343), and BISAP scores (P=0.669; Table 5). In predicting organ failure, the accuracy of pleural effusion volume was similar to that of the CTSI scores (P=0.473), APACHE II scores (P=0.119), and BISAP scores (P=0.980; Table 5), and the accuracy of pulmonary consolidation scores was also similar to that of the CTSI scores (P=0.236), APACHE II scores (P=0.293), and BISAP scores (P=0.612; Table 5). However, the accuracy of the APACHE II scores was significantly superior to that of the CTSI scores in predicting organ failure (P=0.009; Table 5).
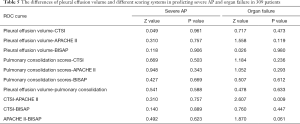
Full table
Table 6 shows the AUCs, best cutoff, sensitivity, specificity, and P value of the pleural effusion volume, pulmonary consolidation scores, and different scoring systems in predicting severe AP and organ failure.
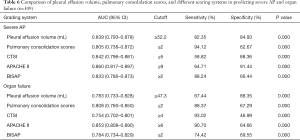
Full table
Pleural effusion volume and pulmonary consolidation scores for evaluating the severity of organ failure
The severity of organ failure was classified into no organ failure, transient organ failure, and persistent organ failure. According to the Kruskal-Wallis H and Student-Newman-Keuls test, the pleural effusion volume (25.4±23.5 mL) in the patients with no organ failure was lower than that (137.4±116.9 mL) of the patients with organ failure (P=0.000). Whereas the pleural effusion volume (156.8±146.8 mL) of patients with persistent organ failure was similar to that (134.2±127.9 mL) of transient organ failure (P=0.134). The pulmonary consolidation scores (0.8±1.0 points) in the patients with no organ failure were lower than those (2.4±1.2 points) of the patients with organ failure (P=0.000). Meanwhile, the pulmonary consolidation scores (2.4±0.8 points) of the patients with persistent organ failure were similar to those (2.4±1.4 points) with transient organ failure (P=0.807) (Tables 4,7).
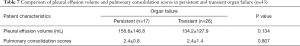
Full table
Pleural effusion volume (r=0.308, P=0.000) and pulmonary consolidation scores (r =0.348, P=0.000) were moderately correlated with the severity of organ failure.
The correlation of pleural effusion volume and pulmonary consolidation scores with death
The mean pleural effusion volume for the patients without death was 39.0±36.0 mL, and it was 144.0±140.3 mL for the patients who died (P=0.000). The mean pulmonary consolidation score for the patients without death was 1.0±1.1 points, and it was 3.0±1.1 points in the patients who died (P=0.000). There was a paucity of data for in-hospital mortality in our series, and this factor was therefore not considered in ROC analysis.
Discussion
In this study, we observed that pleural effusion and pulmonary consolidation were common in CT imaging of AP patients. Bilateral pleural effusion occurred much more often than the left and right types. The pleural effusion volume and pulmonary consolidation scores had a higher correlation with the CTSI and BISAP scores than those of APACHE II scores and the length of hospitalization. Our results demonstrated that pleural effusion volume and the number of consolidation pulmonary lobes are highly correlated with the occurrences of severe AP and organ failure. To our knowledge, this is the first study to evaluate and predict the severity of AP with semi-quantitative pleural effusion and pulmonary consolidation. Our findings may add the value to the use of CT as it may be used to predict the severity of AP earlier than commonly available, especially for severe AP and organ failure.
There are several mechanisms underlying pleural effusion in pancreatitis. One of the mechanisms is transdiaphragmatic lymphatic blockage (3). There involves a possible disruption of the pancreatic duct, leading to the leakage of pancreatic enzymes and the formation of a pancreatic pleural fistula (3). The latter is more likely to occur if the duct disruption is near the posterior retroperitoneum (3,15). Exudation of fluid into the pleural cavity from the subpleural diaphragmatic vessels may also cause pleural effusion (3). The etiology of pulmonary consolidation in AP patients is complex. Abdominal hypertension and systemic inflammatory response syndrome associated with AP are the main causes of early lung injury (7). Pancreatic proteolytic enzymes, along with inflammatory mediators released because of pancreatic injury, play a key role in pulmonary consolidation (3). Pancreatitis-associated protein (PAP) released by the pancreas can mediate lung inflammation through the induction of hepatic TNFalpha expression and subsequent increase in circulating TNFalpha (16). Increased levels of inflammatory markers such as IL-6, TLR4, iNOS, and pulmonary intravascular macrophages play a significant role in lung consolidation (17). Lung microvasculature is also inflamed in AP patients with pulmonary consolidation (17).
Based on the results in previous studies, the prevalence of pleural effusion in the patients with AP was in the range of 4–50% (3,4,6,17). In our study, the prevalence of pleural effusion in the AP patients was calculated to be 39.8%, which was consistent with the reports of other studies. In this study, all three types of pleural effusion were observed, including right, left, and bilateral pleural effusion. However, bilateral pleural effusion was most commonly observed (65%), which was similar to the results of a CT study and a chest X-ray study (4,18). In contrast, Maringhini et al. (19) found that left-sided effusion was observed most frequently (60%) by ultrasonography. The discrepancy in the results among different studies may be due to (I) the low number of cases (n=100) in the previous ultrasonography study; (II) ultrasonography was performed within 3 days of admission in the study by Maringhini et al. (19) while CT examination was performed within a 2-day window of the onset in this study; (III) obese patients were excluded in the ultrasonography study due to the challenges of ultrasound imaging of obese patients.
The prevalence of pulmonary consolidation was 47.9% in this study, which was consistent with the report of another CT study (4,12), whereas it was higher than that by chest radiograph (18). Moreover, in our study, pulmonary consolidation usually occurred in the bilateral lower lobes of the lung, which was consistent with the results of other CT studies (4).
A uniform grading standard for pleural effusion is currently lacking (4,11,18-21). In a previous study, the small, medium, and large effusions were defined as the effusions whose sizes were less than one-third, one-third to two-thirds, and more than two-thirds of the thoracic cage, respectively (20). Moy et al. (21) proposed that the small, medium, or large effusion can be best classified by the anteroposterior quartile and the maximum anteroposterior depth of the clavicle midline in the CT image. Liu et al. (4) measured the thickness of pleural effusion in the axial image and quantified the volume of effusion using pleural effusion/thoracic. The CT volumetry was considered as the gold standard for the volume of pleural effusion (12). Previous studies have reported the prevalence of pulmonary consolidation in AP (3,4,17,18), but none performed quantitative evaluation except for Liu et al. (4). However, the scanning range of CT imaging in their study did not include the whole chest. To our knowledge, ours is the first study to evaluate and predict the severity of AP with semi-quantitative pleural effusion and pulmonary consolidation by using chest CT.
The pleural effusion volume and pulmonary consolidation scores had a higher correlation with the CTSI and BISAP scores than those of APACHE II scores and the length of hospitalization. However, Liu et al. (4) found the pleural effusion/thoracic thickness and pulmonary consolidation/thoracic thickness on CT strongly correlated to EPIC and BISAP scores, and moderately correlated to APACHE II scores and CTSI scores. The reasons for this are as follows: (I) pleural effusion and pulmonary consolidation reflect the thoracic changes, while CTSI focuses on the local pancreatic condition. There are some anatomical channels between the thorax and the abdomen (5). So the inflammation of the pancreas and surrounding areas may go into the chest along these anatomical pathways; (II) the BISAP score was calculated by pleural effusion and other methods (10); (III) the APACHE II scores directly focus on the overall manifestation of the patients (9); (IV) the days of hospital stay duration may not accurately represent morbidity because other factors may prolong the length of stay (22). Therefore, the pleural effusion volume, pulmonary consolidation scores, and the above three scoring systems can be correlated, and thus be used to evaluate the AP severity from different aspects.
Previous studies have compared the prevalence of pleural effusion in mild AP with that in severe AP and reported that pleural effusions are observed most often in severe AP (4,23). Maringhini et al. (19) used ultrasonography and multivariate analysis to demonstrate that pleural effusion is an accurate, independent predictor of severity. Liu et al. (4) compared the ratios of pulmonary consolidation with thoracic thickness on CT between mild and severe AP and indicated that bilateral pulmonary consolidation can indicate severe AP. However, they classified AP into 2 degrees of severity: severe and mild. According to the 2012 revised Atlanta classification, AP was defined into 3 degrees: mild, moderately severe, and severe AP. Raghu et al. (18) claimed the development of consolidation correlates with the occurrence of respiratory failure. Based on the 2012 revised Atlanta classification, our results revealed that pleural effusion volume and consolidation pulmonary lobes are highly correlated with the occurrences of severe AP and organ failure in the early stage, and that they have the accuracy to CTSI, APACHE II, and BISAP scores. This is the first study to predict the occurrences of the severe AP and organ failure with semi-quantitative pleural effusion and pulmonary consolidation by using chest CT.
We found that pleural effusion volume and consolidation pulmonary lobes demonstrated a moderate correlation with the severity of organ failure, and no significant differences were present in the pleural effusion volume and consolidation pulmonary lobes between transient and persistent organ failure. Huang et al. (13) revealed that the exudation of pleural effusion increased within 1 week but declined at time intervals of within 2 weeks and longer. The early phase of AP lasts 1 week, and the severity of AP primarily depends on the presence and duration of organ failure due to a systemic inflammatory response (2). Dombernowsky et al. (12) suggested that acute lung injury is possibly, associated with systemic inflammation. So, the duration of organ failure is related to the duration of systemic inflammation and not the presence of pleural effusion and pulmonary consolidation. In this study, we also discovered that the increased pleural effusion volume and pulmonary consolidation scores were associated with the occurrence of death.
In addition to these promising findings, some limitations to this study should also be addressed. Firstly, there was a variable interval between CT examination and the onset of AP. The variation of the interval can influence the change of CTSI. To minimize the variation of the interval, the CT scan was performed within 48 hours of admission. Secondly, the APACHE II score was obtained from several nurses and physicians. However, the calculation of the APACHE II score by different professionals might have led to variations between the different observers (9,24). However, this variation had little influence on the evaluation of pleural effusion and pulmonary consolidation from the CT imaging and on the main conclusions of the study.
Conclusions
In summary, pleural effusion and pulmonary consolidation in the CT imaging of AP patients could be commonly observed and thus are correlated to the severity of AP. The volume of pleural effusion and the lobe number of pulmonary consolidations can act as early predictors of severe AP and organ failure.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study (including contrast-enhanced CT) gained approval from the Institutional Review Board in Panzhihua Central Hospital. All the patients signed informed consent before undergoing CT scans.
References
- Xie CL, Zhang M, Chen Y, Hu R, Tang MY, Chen TW, Xue HD, Jin ZY, Zhang XM. Spleen and splenic vascular involvement in acute pancreatitis: an MRI study. Quant Imaging Med Surg 2018;8:291-300. [Crossref] [PubMed]
- Chen C, Huang Z, Li H, Song B, Yuan F. Evaluation of extrapancreatic inflammation on abdominal computed tomography as an early predictor of organ failure in acute pancreatitis as defined by the revised Atlanta classification. Medicine (Baltimore) 2017;96:e6517. [Crossref] [PubMed]
- Kumar P, Gupta P, Rana S. Thoracic complications of pancreatitis. JGH Open 2018;3:71-9. [Crossref] [PubMed]
- Liu D, Song B, Huang ZX, Yuan F, Li WM. The value of chest CT features evaluating the severity and prognosis for acute pancreatitis. Sichuan Da Xue Xue Bao Yi Xue Ban 2013;44:319-22. [PubMed]
- Xu H, Ebner L, Jiang S, Wu Y, Christe A, Zhang S, Zhang X, Luo Z, Tian F. Retrocrural space involvement on computed tomography as a predictor of mortality and disease severity in acute pancreatitis. PLoS One 2014;9:e107378. [Crossref] [PubMed]
- Raghuwanshi S, Gupta R, Vyas MM, Sharma R. CT Evaluation of Acute Pancreatitis and its Prognostic Correlation with CT Severity Index. J Clin Diagn Res 2016;10:TC06-11. [PubMed]
- Sun H, Zuo HD, Lin Q, Yang DD, Zhou T, Tang MY, Wang YXJ, Zhang XM. MR imaging for acute pancreatitis: the current status of clinical applications. Ann Transl Med 2019;7:269. [Crossref] [PubMed]
- Wang YX, Chen S, Morcos SK. Contrast-enhanced CT in acute pancreatitis. Br J Radiol 1999;72:1029. [Crossref] [PubMed]
- Tang W, Zhang XM, Xiao B, Zeng NL, Pan HS, Feng ZS, Xu XX. Magnetic resonance imaging versus Acute Physiology And Chronic Healthy Evaluation II score in predicting the severity of acute pancreatitis. Eur J Radiol 2011;80:637-42. [Crossref] [PubMed]
- Senapati D, Debata PK, Jenasamant SS, Nayak AK, Gowda S M, Swain NN. A prospective study of the Bedside Index for Severity in Acute Pancreatitis (BISAP) score in acute pancreatitis: an Indian perspective. Pancreatology 2014;14:335-9. [Crossref] [PubMed]
- Teichgräber UK, Hackbarth J. Sonographic Bedside Quantification of Pleural Effusion Compared to Computed Tomography Volumetry in ICU Patients. Ultrasound Int Open 2018;4:E131-5. [Crossref] [PubMed]
- Dombernowsky T, Kristensen MØ, Rysgaard S, Gluud LL, Novovic S. Risk factors for and impact of respiratory failure on mortality in the early phase of acute pancreatitis. Pancreatology 2016;16:756-60. [Crossref] [PubMed]
- Huang H, Chen W, Tang G, Liang Z, Qin M, Qin M, Tang Y, Qin H, Chang R. Optimal timing of contrast-enhanced computed tomography in an evaluation of severe acute pancreatitis-associated complications. Exp Ther Med 2019;18:1029-38. [PubMed]
- Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS. Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013;62:102-11. [Crossref] [PubMed]
- Pandey S, Shetty SA, Janarthanan K, Balalakshmoji D, Sen KK, Leelakrishnan V. Pancreatico-pleural and bronchial fistulae and associated pseudocysts: case series. JOP 2014;15:478-84. [PubMed]
- Folch-Puy E, García-Movtero A, Iovanna JL, Dagorn JC, Prats N, Vaccaro MI, Closa D. The pancreatitis-associated protein induces lung inflammation in the rat through activation of TNFalpha expression in hepatocytes. J Pathol 2003;199:398-408. [Crossref] [PubMed]
- Vrolyk V, Wobeser BK, Al-Dissi AN, Carr A, Singh B. Lung Inflammation Associated With Clinical Acute Necrotizing Pancreatitis in Dogs. Vet Pathol 2017;54:129-40. [Crossref] [PubMed]
- Raghu MG, Wig JD, Kochhar R, Gupta D, Gupta R, Yadav TD, Agarwal R, Kudari AK, Doley RP, Javed A. Lung complications in acute pancreatitis. JOP 2007;8:177-85. [PubMed]
- Maringhini A, Ciambra M, Patti R, Randazzo MA, Dardanoni G, Mancuso L, Termini A, Pagliaro L. Ascites, pleural, and pericardial effusions in acute pancreatitis.A prospective study of incidence, natural history, and prognostic role. Dig Dis Sci. 1996;41:848-52. [Crossref] [PubMed]
- Mironov O, Ishill NM, Mironov S, Vargas HA, Zheng J, Moskowitz CS, Sonoda Y, Papas RS, Chi DS, Hricak H. Pleural effusion detected at CT prior to primary cytoreduction for stage III or IV ovarian carcinoma: effect on survival. Radiology 2011;258:776-84. [Crossref] [PubMed]
- Moy MP, Levsky JM, Berko NS, Godelman A, Jain VR, Haramati LB. A new, simple method for estimating pleural effusion size on CT scans. Chest 2013;143:1054-9. [Crossref] [PubMed]
- Meyrignac O, Lagarde S, Bournet B, Mokrane FZ, Buscail L, Rousseau H, Otal P. Acute Pancreatitis: Extrapancreatic Necrosis Volume as Early Predictor of Severity. Radiology 2015;276:119-28. [Crossref] [PubMed]
- Uchikov AP, Shipkov HD, Markova DI. Pleural effusions in acute pancreatitis. Folia Med (Plovdiv) 2000;42:34-6. [PubMed]
- Peng R, Zhang XM, Ji YF, Chen TW, Yang L, Huang XH, Chi XX. Pancreatic duct patterns in acute pancreatitis: a MRI study. PLoS One 2013;8:e72792. [Crossref] [PubMed]


