
Lung cancer screening: how do we make it better?
Lung cancer screening with low-dose computed tomography (LDCT) has been available as a Medicare covered test in the United States since February 2015, for asymptomatic patients age 55 to 77 years with a history of smoking at least 30 pack-years and, if a former smoker, having quit within the previous 15 years (1). Given that the National Comprehensive Cancer Network (NCCN) issued guidelines for lung cancer screening in October, 2011 (2), the concept has been advocated for close to a decade. The U.S. Preventive Services Task Force (USPSTF) recommends annual screening for lung cancer with LDCT in persons at high risk for lung cancer based on age and smoking history (3).
A concern with current guidelines based on the National Lung Screening Trial (NLST) is the low annual detection rate of 1%, based on age and pack-years alone. Only 50% of all those who will develop lung cancer would be eligible for screening by these criteria (3). The detection rate will increase if other risk factors for lung cancers and validated risk prediction models are included in the selection criteria of patients (3,4). Most trials have selected participants who are considered to be at high risk for lung cancer on the basis of smoking history. However, the benefits from screening could be improved if it were possible to more precisely identify a high-risk population. Many risk prediction models of varying degrees of complexity that incorporate factors in addition to smoking have been proposed to better identify high-risk groups (5-14), including our simple 4 factor model (15).
In 2013, the group from the Division of Cancer Epidemiology and Genetics of the NCI published a more specific risk-prediction model for lung-cancer mortality by taking into account more factors than the NLST entry criteria. By applying this model to the NLST population, they showed that the population at the high end of the risk spectrum had more benefits and less harm. The number needed to screen could be reduced from 320 in the NLST to 161, and false positive screening CT could be cut from more than 100 to around 65 for every prevented lung cancer death. It was suggested that this model, if validated in the general population, could improve LDCT screening benefits with reduced harm of false positives (12). This same group, now from the Intramural Research Group of the National Cancer Institute, recently published a new selection model for lung cancer screening based on life gained, versus risk-based selection (16). This is a heavily modeled study with many assumptions used to calculate life gained from LDCT. A few examples include: (I) the model is heavily based on the NLST screening protocol (3 annual LDCT/5 year follow-up) not real life (annual LDCT until patient ages out or has quit smoking >15 years), (II) NLST was used for modeling non NLST eligible subjects (age 40–55, smokers <30 pack years) and (III) the model assumes LDCT does not affect non-lung cancer mortality (NLST showed 6.9% reduction in all-cause mortality in LDCT group). Using their life-gained-based selection model in US ever smokers aged 40–84 years and selecting 8.3 million ever smokers to match the number selected by USPSTF criteria, total life expectancy is increased by approximately 25,600 years compared to a risk-based selection, but approximately 2,400 fewer lung cancer deaths are prevented. The authors conclude that a life-gained-based selection could maximize the benefits of LDCT screening in the US by including ever smokers who have both high lung cancer risk and long-life expectancy.
More important than the selection method for LDCT lung cancer screening, is the reality that so few eligible patients are actually getting screened in the US. Increasingly complicated selection criteria will further hinder efforts to increase recruitment and lung screening uptake. We need to make it easier to get eligible patients to the starting line. The lack of enthusiasm for all of the above referenced “better and improved” risk prediction models re-enforces the point.
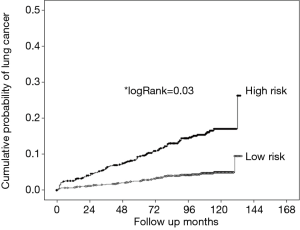
There are options. We could relax the USPSTF criteria—decrease eligible age to 40 and eliminate the 15-year quit rule. Concerns about opening the floodgates to LDCT are probably unrealistic given the general lack of enthusiasm to date of those already eligible. A better option, consistent with our desire to keep eligibility criteria simple, would be to use COPD (airflow obstruction and/or emphysema) as an eligibility factor. We have shown that COPD is a very important and robust predictor of lung cancer risk (17-22). For example, we developed and validated the COPD Lung Cancer Screening Score (COPD-LUCSS) that incorporated age, body mass index and presence of emphysema. Two COPD-LUCSS categories were proposed, with the high-risk group having a 3.5-fold increased lung cancer risk (Figure 1) (21). Combining COPD with NLST criteria to select LDCT screening candidates would result in higher lung cancer detection rates and a lower number of cancers missed (19). There is also evidence that COPD patients more enthusiastically support lung cancer screening (23).
In conclusion, there are many options for selecting patients for LDCT lung cancer screening. We agree with Cheung et al. that considering life expectancy in cancer screening is important. Whether an individualized life gained calculator would realistically be implemented is debatable. We also agree that for thresholds that enable screening of similar numbers of ever smokers, risk-based selection maximizes the number of lung cancer deaths averted and life-gained-based selection maximizes the life years gained in the population. However, in the context of overcoming the low turnout for LDCT lung cancer screening, does this really matter? Also, as the authors have pointed out, a shift to life gained based selection would compound problems related to the time, effort, feasibility and reliability of the collection of information on demographic measurements; risk behaviors; and multiple health conditions, many of which may not be readily available in the medical record. Finally, prospective studies would be needed, to determine whether a population can be readily identified using risk models in which screening would have greater benefit than the 20 percent lung cancer-mortality benefit identified in the NLST. In addition, how to implement and operationalize individual risk-based screening remains a major challenge, particularly given the reality of poor participation in LDCT lung cancer screening nationally.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Decision Memo for Screening for Lung Cancer with Low Dose Computed Tomography (LDCT). Available online: http://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274
- NCCN Clinical Practice Guidelines in Oncology. Lung cancer screening. Version 1, 2014. Available online: http://www.nccn.org/professionals/physician_gls/pdf/lung_screening.pdf
- U.S. Preventive Services Task Force. Draft Research Plan: Lung Cancer: Screening. Rockville, MD: U.S. Preventive Services Task Force, 2018. Available online: www.uspreventiveservicestaskforce.org/Page/Document/draft-research-plan/lung-cancer-screening1
- National Lung Screening Trial Research Team. Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM, Sicks JD. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Katki HA, Kovalchik SA, Berg CD, Cheung LC, Chaturvedi AK. Development and Validation of Risk Models to Select Ever-Smokers for CT Lung Cancer Screening. JAMA 2016;315:2300-11. [Crossref] [PubMed]
- Raji OY, Duffy SW, Agbaje OF, Baker SG, Christiani DC, Cassidy A, Field JK. Predictive accuracy of the Liverpool Lung Project risk model for stratifying patients for computed tomography screening for lung cancer: a case-control and cohort validation study. Ann Intern Med 2012;157:242-50. [Crossref] [PubMed]
- Cronin KA, Gail MH, Zou Z, Bach PB, Virtamo J, Albanes D. Validation of a model of lung cancer risk prediction among smokers. J Natl Cancer Inst 2006;98:637-40. [Crossref] [PubMed]
- Etzel CJ, Bach PB. Estimating individual risk for lung cancer. Semin Respir Crit Care Med 2011;32:3-9. [Crossref] [PubMed]
- Spitz MR, Hong WK, Amos CI, Wu X, Schabath MB, Dong Q, Shete S, Etzel CJ. A risk model for prediction of lung cancer. J Natl Cancer Inst 2007;99:715-26. [Crossref] [PubMed]
- Tammemagi CM, Pinsky PF, Caporaso NE, Kvale PA, Hocking WG, Church TR, Riley TL, Commins J, Oken MM, Berg CD, Prorok PC. Lung cancer risk prediction: Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial models and validation. J Natl Cancer Inst 2011;103:1058-68. [Crossref] [PubMed]
- Lowry KP, Gazelle GS, Gilmore ME, Johanson C, Munshi V, Choi SE, Tramontano AC, Kong CY, McMahon PM. Personalizing annual lung cancer screening for patients with chronic obstructive pulmonary disease: A decision analysis. Cancer 2015;121:1556-62. [Crossref] [PubMed]
- Kovalchik SA, Tammemagi M, Berg CD, Caporaso NE, Riley TL, Korch M, Silvestri GA, Chaturvedi AK, Katki HA. Targeting of low-dose CT screening according to the risk of lung-cancer death. N Engl J Med 2013;369:245-54. [Crossref] [PubMed]
- Tammemägi MC, Katki HA, Hocking WG, Church TR, Caporaso N, Kvale PA, Chaturvedi AK, Silvestri GA, Riley TL, Commins J, Berg CD. Selection criteria for lung-cancer screening. N Engl J Med 2013;368:728-36. [Crossref] [PubMed]
- Katki HA, Kovalchik SA, Petito LC, Cheung LC, Jacobs E, Jemal A, Berg CD, Chaturvedi AK. Implications of Nine Risk Prediction Models for Selecting Ever-Smokers for Computed Tomography Lung Cancer Screening. Ann Intern Med 2018;169:10-9. [Crossref] [PubMed]
- Wilson DO, Weissfeld J. A simple model for predicting lung cancer occurrence in a lung cancer screening program: The Pittsburgh Predictor. Lung Cancer 2015;89:31-7. [Crossref] [PubMed]
- Cheung LC, Berg CD, Castle PE, Katki HA, Chaturvedi AK. Life-gained-based versus risk-based selection of smokers for lung cancer screening. Ann Intern Med 2019. [Crossref] [PubMed]
- Wilson DO, Weissfeld JL, Balkan A, Schragin JG, Fuhrman CR, Fisher SN, Wilson J, Leader JK, Siegfried JM, Shapiro SD, Sciurba FC. Association of radiographic emphysema and airflow obstruction with lung cancer. Am J Respir Crit Care Med 2008;178:738-44. [Crossref] [PubMed]
- de Torres JP, Bastarrika G, Wisnivesky JP, Alcaide AB, Campo A, Seijo LM, Pueyo JC, Villanueva A, Lozano MD, Montes U, Montuenga L, Zulueta JJ. Assessing the relationship between lung cancer risk and emphysema detected on low-dose CT of the chest. Chest 2007;132:1932-8. [Crossref] [PubMed]
- Sanchez-Salcedo P, Wilson DO, de-Torres JP, Weissfeld JL, Berto J, Campo A, Alcaide AB, Pueyo J, Bastarrika G, Seijo LM, Pajares MJ, Pio R, Montuenga LM, Zulueta JJ. Improving selection criteria for lung cancer screening. The potential role of emphysema. Am J Respir Crit Care Med 2015;191:924-31. [Crossref] [PubMed]
- Yong PC, Sigel K, de-Torres JP, Mhango G, Kale M, Kong CY, Zulueta JJ. Wilson D6, Brown SW, Slatore C, Wisnivesky J. The effect of radiographic emphysema in assessing lung cancer risk. Thorax 2019;74:858-64. [Crossref] [PubMed]
- de-Torres JP, Wilson DO, Sanchez-Salcedo P, Weissfeld JL, Berto J, Campo A, Alcaide AB, García-Granero M, Celli BR, Zulueta JJ. Lung cancer in patients with chronic obstructive pulmonary disease. Am J Resp Crit Care Med 2015;191:285-91. [Crossref] [PubMed]
- Carr LL, Jacobson S, Lynch DA, Foreman MG, Flenaugh EL, Hersh CP, Sciurba FC, Wilson DO, Sieren JC, Mulhall P, Kim V, Kinsey CM, Bowler RP. Features of COPD as Predictors of lung cancer. Chest 2018;153:1326-35. [Crossref] [PubMed]
- Pallin M, Walsh S, O'Driscoll MF, Murray C, Cahalane A, Brown L, Carter M, Mitchell P, McDonnell TJ, Butler MW. Overwhelming support among urban Irish COPD patients for lung cancer screening by low-dose CT scan. Lung 2012;190:621-8. [Crossref] [PubMed]


