Diffusion-weighted imaging (DWI) ischemic volume is related to FLAIR hyperintensity-DWI mismatch and functional outcome after endovascular therapy
Introduction
Acute ischemic stroke (AIS) is a cerebrovascular disease that is seriously harmful to human life and has high mortality and disability rates (1,2). It is very important to predict outcomes early in the treatment and rehabilitation of stroke patients. Currently available studies have reported that valuable imaging markers that can predict stroke patient outcomes include National Institutes of Health Stroke Scale (NIHSS) scores, Alberta Stroke Program Early Computed Tomography Scores (ASPECTS), and collateral circulation, and diffusion-weighted imaging (DWI) infarct volume (3-7). Some studies have suggested that AIS patients with larger infarct volumes have worse clinical outcomes following intravenous thrombolysis (8,9). Research investigating the changes in DWI volume after thrombolysis has demonstrated a consistent correlation with clinical outcome (10,11). The quantification of infarction evolution has been considered capable of determining the efficacy of therapy and may also be used as a surrogate outcome measure for stroke trials (12,13).
Fluid-attenuated inversion recovery (FLAIR) and DWI sequences are part of acute stroke magnetic resonance imaging (MRI) protocols in institutions using MR imaging as a first-line diagnostic tool (14-16). The earliest MRI finding, occurring within minutes after occlusion of cerebral blood flow, is restricted diffusion on DWI, correlating with cytotoxic edema in ATP-deprived cells (17-19). On the other hand, there is a gradual increase in FLAIR signal intensity in the infarcted region, which may not be observed until a few hours after abnormal DWI signal appears. FLAIR vascular hyperintensities (FVH) appear as a circular or serpentine brightening in the brain parenchyma or the cortical surface bordering the subarachnoid space (20). FVH precedes DWI abnormalities (21) and can be seen beyond the boundaries of the DWI lesion (22). Some studies have reported that FVH may provide prognostic information (14). FVH-DWI mismatch focuses on FVH beyond the boundaries of the cortical DWI lesion, ignoring FVH adjacent to the DWI lesion. Currently, available studies have generally focused on whether the FVH-DWI mismatch predicts PWI-DWI mismatch or ischemic penumbra (23). The associations between DWI volume and FVH-DWI mismatch, as well as those between the effect of DWI volume and DWI volume growth on functional outcome, have not been reported.
Therefore, in this study, we sought to assess whether DWI volume and DWI volume growth were associated with FVH-DWI mismatch, and the effect of DWI volume and DWI volume growth on functional outcome at 3 months after acute stroke in patients who received thrombectomy therapy. We hypothesized that DWI volume and DWI volume growth would be associated with FVH-DWI mismatch, and could provide functional outcome information in acute stroke patients.
Methods
Subjects and clinical data
The hospital review board of Nanjing Medical University approved the study protocol. The prospective registry of AIS patients was evaluated with data obtained at the Nanjing First Hospital between January 2017 and March 2019. All patients were treated according to the guidelines for managing AIS (24). Patients who met the criteria for intravenous thrombolytic therapy received intravenous thrombolysis (Alteplase; rt-PA) within 4.5 h of stroke onset after a CT scan, and an MRI examination immediately followed. If a large vessel [the middle cerebral artery (MCA) -M1 or/and the internal carotid artery (ICA)] occlusion was found on magnetic resonance angiography (MRA) within 6 h of stroke onset, thrombectomy was immediately performed. The patients included in the present study presented the following: (I) a first-ever acute anterior circulation stroke or a previous stroke with hemiplegia sequelae that did not affect the neurological score; (II) acute stroke saw ≤6 h within symptom onset; (III) CT scan after admission; (IV) pretreatment MRI with DWI, FLAIR, and MRA; (V) receiving thrombectomy therapy; (VI) follow-up MRI was performed within 24 h after thrombectomy therapy; and (VII) a clinical follow-up with a modified Rankin scale (mRS) score at 3 months. The exclusion criteria were as follows: (I) cerebral hemorrhage, tumor, or trauma detected on a CT scan; (II) any contraindication for MRI; (III) any missing mRS at 3 months after stroke; (IV) refusal of thrombectomy therapy; and (V) any MRI or DSA that could not be evaluated due to a motion artifact.
Age, gender, homocysteine levels (blood test level >15 µmol/L), NIHSS score (>5 score) at admission, history of hypertension (>140/90 mmHg), diabetes mellitus [fasting plasma glucose ≥126 mg/dL (7.00 mmol/L) or 2-h plasma glucose after a 75-g oral glucose tolerance test ≥200 mg/dL (11.1 mmol/L)], hyperlipidemia [blood serum total cholesterol >150 mg/dL (1.70 mmol/L)/triglyceride >220 mg/dL (5.72 mmol/L)/low density lipoprotein-cholesterol >140 mg/dL (3.64 mmol/L)], and atrial fibrillation, were collected. The functional outcome at 3 months was assessed using the mRS. A good functional outcome was defined as an mRS score ≤ of 2 at 3 months (25). All patients had written informed consent signed by themselves or their family members prior to their participation in this study. The study was approved by the local ethics committee of the Nanjing Medical University.
MRI protocol
All patients underwent an MRI examination before thrombectomy and after thrombectomy therapy within 24 h. The MRI data were acquired using a 3T MR scanner (Ingenia, Philips Medical Systems, Best, the Netherlands) with an 8-channel receiver array head coil. The standard MRI scanning protocol included FLAIR, DWI, and MRA. The imaging parameters for the FLAIR were as follows: 7,000/120 ms TR/TE, 356*151 matrix, 230*230 mm2 field of view (FOV), 90° FA, 18 slices, 6-mm-thick sections, and 1.3 mm intersection gap. The imaging parameters for the DWI were as follows: 2,245/90 ms [repetition time (TR)/echo time (TE)], bmax=1,000 s/mm2, 140*109 matrix, 210*210 mm2 FOV, 6-mm-thick sections, and 1.3 mm intersection gap. The imaging parameters for the 3D-MRA were as follows: fast field echo (FFE) sequence, 4.9/1.82 ms TR/TE, 528*531 matrix, 330*330 mm2 FOV, and 1.2-mm-thick sections.
Image analysis
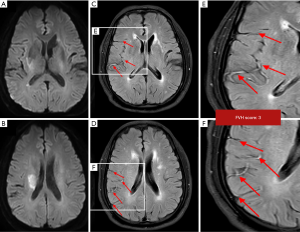
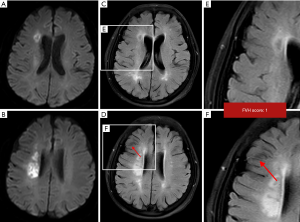
Two experienced neuroradiologists (H.Y.C and M.Y.P) who were blinded to the clinical data, independently evaluated these images. In cases of discrepant assessment results between the two readers, images were reviewed, and a consensus was established. DWI volume was measured using a Philips MRI scanner postprocessing workstation. DWI volume was automatically calculated after delineating the region of interest of infarct volume on DWI images. DWI volume growth was defined as DWI volume on follow-up minus DWI volume on admission. FVH was defined as focal, tubular, or serpentine hyperintensity present in the subarachnoid space relative to cerebrospinal fluid with a typical arterial course (20). The FVH score was assessed according to their spatial distribution in the ASPECTS of cortical areas (insula, M1-M6) (26). FVH-DWI mismatch was assessed based on axial FLAIR and DWI images, and the FVH-DWI mismatch was considered present when FVHs extended beyond the boundaries of the cortical DWI lesion (i.e., when ≥1 FVH was facing the isointense cortex on DWI). No FVH-DWI mismatch was defined as no FVH or all FVHs facing the hyperintense cortex on DWI.
Two experienced interventional neuroradiologists (H.B.S and B.X.Z) who were blinded to the clinical information assessed the baseline angiography data of stroke patients. The collateral grading score was evaluated using the American Society of Interventional and Therapeutic Neuroradiology (ASITN) scoring system (0 = no collaterals, 4 = complete and rapid collateral perfusion of the ischemic territory) (27). Good collateral status was defined as an ASITN grade of 3–4 (28).
Statistical analysis
All statistical analyses were conducted using commercially available software (SPSS for Windows, version 19.0; SPSS). Continuous data are described as the mean ± SD and were compared using independent-samples t-test or the Mann-Whitney U test, while categorical variables are presented as a number (percentage), and were compared using chi-squared test or Fisher’s exact tests. P<0.05 was considered to indicate statistical significance. Receiver operating characteristic (ROC) curve analysis was used to assess the value of DWI volume to predict functional outcome in patients with AIS after thrombectomy.
Results
Comparison of FVH/DWI mismatch and no FVH/DWI mismatch with acute stroke patients
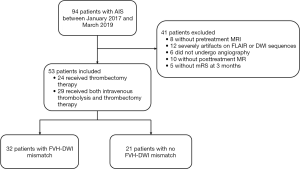
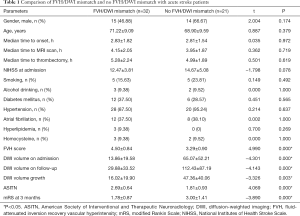
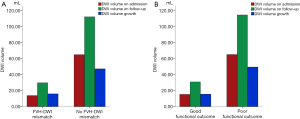
Among the 94 patients in the study, 53 (29 males and 24 females; aged: mean ± SD, 70.06±9.341 years old, range, 41–80 years old) fulfilled the inclusion criteria. Forty-one patients were excluded (8 patients, no pretreatment MRI; 12 patients, severe artifacts on FLAIR or DWI sequences; 6 patients, did not undergo angiography; 10 patients, no posttreatment MR; 5 patients, no mRS at 3 months). Out of the 53 patients, 24 (45.28%) received thrombectomy therapy, and 29 (54.72%) received both intravenous thrombolysis and thrombectomy therapy. Thirty-two (60.38%) had FVH-DWI mismatch, with the interobserver agreement for FVH-DWI mismatch being k=0.95 (95% CI, 0.91–0.99) (Figure 1). As shown in Table 1, compared to the without FVH-DWI mismatch group, the FVH-DWI mismatch group had a smaller DWI volume on admission (13.86±19.58 vs. 65.07±52.21; t=−4.301, P=0.000), smaller DWI volume on follow-up (29.88±33.52 vs. 112.43±87.19; t=−4.143, P=0.000), lower DWI volume growth (16.02±19.90 vs. 47.36±40.06; t=−3.326, P=0.003), higher FVH scores (4.50±0.84 vs. 3.29±0.90; t=4.990, P=0.000), and a higher ASITN (2.69±0.64 vs. 1.81±0.93; t=4.069, P=0.000) (Figures 2-4). The 3-month outcome was better in patients with FVH-DWI mismatch (1.78±0.87) than that in patients without FVH-DWI mismatch (3.00±1.41) (t=−3.890; P=0.000) (Table 1).
Full table
Comparison of good functional outcome and poor functional outcome with acute stroke patients
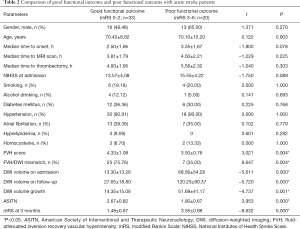
The analysis of 53 AIS patients revealed that 33 patients (62.26%) had a good functional outcome (mRS at 3 months 0–2), and 20 (37.74%) had a poor functional outcome (mRS at 3 months 3–6). The good functional outcome group had a smaller DWI volume on admission (13.30±13.26 vs. 68.56±54.28; t=−5.611, P=0.000), smaller DWI volume on follow-up (27.65±18.80 vs. 120.25±90.37; t=−5.720, P=0.000), and lower DWI volume growth (14.35±15.06 vs. 51.69±41.17; t=−4.737, P=0.001) than those of the poor functional outcome group (Figure 4). In addition, the good functional outcome group had a higher FVH score (4.33±1.08 vs. 3.50±0.76; t=3.021; P=0.004), a higher FVH-DWI mismatch ratio (75.76% vs. 35%; t=8.647; P=0.004), and a higher ASITN grade (2.67±0.82 vs. 1.80±0.67; t=3.953; P=0.000) than those of the poor functional outcome group. There were no significant differences in gender, hypertension, diabetes mellitus, hyperlipidemia, atrial fibrillation, or homocysteine levels between the two groups (P>0.05) (Table 2).
Full table
ROC analysis for the functional outcome with acute stroke patients
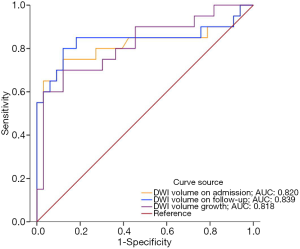
ROC analysis showed the findings for the DWI volume on admission, DWI volume on follow-up, and DWI volume growth to predict functional outcome in stroke patients were 0.820 (95% CI, 0.679–0.962), 0.839 (95% CI, 0.702–0.977), and 0.818 (95% CI, 0.694–0.943) respectively. When the optimal cut-off value of DWI volume on admission was 33.50 mL, the sensitivity and specificity for predicting functional outcome were 65% and 96.97%, respectively; DWI volume on follow-up was 48.6 mL, and had the sensitivity and specificity of 80% and 87.88%, respectively; DWI volume growth was 22.25 mL, and had a sensitivity and specificity of 70% and 87.88%, respectively (Figure 5).
Discussion
From our analysis of patients with acute stroke, we found that FVH-DWI mismatch had a smaller DWI volume on admission, smaller DWI volume on follow-up, lower DWI volume growth, and better functional outcome than those of patients with FVH-DWI mismatch. In addition, patients with good functional outcomes had a higher FVH-DWI mismatch ratio, smaller DWI volume on admission, smaller DWI volume on follow-up, and lower DWI volume growth than those of patients in the poor functional outcome group. DWI volume on admission, DWI volume on follow-up, and DWI volume growth had higher sensitivity and specificity in predicting functional outcome in stroke patients. Comprehensively evaluating DWI volume on admission, DWI volume on follow-up and DWI volume growth could provide the prognostic information of acute stroke patients after thrombectomy.
Although the mechanisms underlying this MRI sign and its clinical implications have been controversial, FVH is considered an emergent radiographic marker, which likely represents disordered or sluggish blood flow and is often observed in acute stroke with large vessel occlusion. Slow flow and stasis cause a high signal on FLAIR, which contrasts the normal flow void phenomenon of arteries. Proximal FVH in the MCA territory most often represents thrombus, while distal FVH is likely to represent slow blood flow (29). FVH may represent slow collateral flow effective in maintaining perfusion to penumbral regions, restricting the progress of ischemic lesions, and improving outcome (26,30). Conversely, FVH also seems to correspond to a perfusion deficit, larger lesions, and poor outcome (31,32). This stark discrepancy may be the result of different FVH methods being used, the heterogeneity of samples, or varying symptom-to-MR imaging times. Shang et al. demonstrated that FVH was associated with an unfavorable outcome within 6 hours to 14 days of onset, while distal FVH may be favorable beyond 14 days of onset in MCA infarction (33). At present, there are many methods to evaluate the extent of FVH (30-32,34), each method has its drawbacks, and the most reliable method has not yet been identified. FVH-DWI mismatch focuses on FVHs beyond the boundaries of the cortical DWI lesion and ignores FVHs adjacent to the DWI lesion. There are several advantages to using FVH-DWI mismatch when evaluating FVH extent (35): it is a reproducible method, it does not require gadolinium contrast, and it is easily and directly assessable by the naked eye without the need for postprocessing. In our study, patients with FVH-DWI mismatch had a higher FVH score and a higher ASITN than those of patients with FVH-DWI mismatch, which is consistent with previous research (36). This research demonstrated that FVH is independently associated with better collateral grades (36). There is accumulating evidence indicating that FVH distal to an arterial occlusion represents good collaterals in the early time window. AIS usually involves hemodynamic impairment and slow retrograde collateral leptomeningeal blood flow. As previously described, the FVH score reflects the number of leptomeningeal collateral vessels recruited during the acute disruption of blood flow in the MCA. Thus, the greater the FVH score, the more collateral vessels appear on FLAIR, the higher the rate of the FVH-DWI mismatch. In this study, patients with FVH-DWI mismatch had more abundant collateral circulation, and greater collateral circulation was associated with better functional outcomes.
Good collateral vessels can improve the odds of achieving a larger volume of surviving brain tissue by sustaining the ischemic penumbra (37,38) and are associated with better clinical outcomes (39). In our study, we compared the association between DWI volume and FVH-DWI mismatch. We found that FVH-DWI mismatch patients had a smaller DWI volume on admission, smaller DWI volume on follow-up, and lower DWI volume growth than those of patients without FVH-DWI mismatch. This finding is consistent with Lee et al. (40), who proposed that patients with more prominent distal hyperintense vessels had smaller initial and 24-hour ischemic lesion volumes. In contrast, Hohenhaus et al. (41) suggested that patients with FVH >4 had larger initial DWI lesions and final infarct volume. The reason for some of the differences among studies may be the use of different FVH scoring methods. In addition, we also found that DWI volume on admission, DWI volume on follow-up, and DWI volume growth in good functional outcome groups were smaller than those in patients with poor functional outcomes. DWI lesion volume has been shown to influence the response to thrombectomy therapy; a large DWI lesion on admission is recognized as a useful marker for poor treatment response (42,43). A large DWI infarct volume on admission (>70 mL) is one of the more established imaging markers for poor clinical outcome in acute stroke (42,44-46). Certain studies have suggested that a DWI infarct volume of >70–100 mL represents a malignant profile that has a higher risk of a poor outcome (8,9). In our study, ROC analysis showed that when the optimal cut-off value of DWI volume on admission was 33.50 mL, the sensitivity and specificity for predicting functional outcome was 65% and 96.97%, respectively; DWI volume on follow-up was 48.6 mL, and had a sensitivity and specificity of 80% and 87.88%, respectively; DWI volume growth was 22.25 mL, and had a sensitivity and specificity of 70% and 87.88% respectively. Thus, DWI volume on admission, DWI volume on follow-up, and DWI volume growth could be clinically useful markers of functional outcome.
Despite these informative findings, our study has some limitations that should be addressed. First, the sample size was rather small, and although many AIS patients in the early stage were included, many heterogeneous cases were excluded after rigorous screening. Second, this was a single-center study. Moreover, due to ethical considerations, we performed the MRI scanning immediately after intravenous thrombolysis in patients who qualified for intravenous thrombolysis after CT scanning, without ruling out the effect of intravenous thrombolysis on the MRI. Finally, our results cannot be generalized to all acute stroke patients, especially those who do not undergo thrombectomy therapy. A study of larger sample size with the inclusion of stroke patients with different therapies from many centers should be conducted in the future.
Conclusions
Smaller DWI volume on admission, DWI volume on follow-up, and DWI volume growth were more likely to cause FVH-DWI mismatch, and usually had a good functional outcome with stroke patients. Comprehensively evaluating DWI volume on admission, DWI volume on follow-up and DWI volume growth could provide the prognostic information of acute stroke patients after thrombectomy.
Acknowledgments
Funding: This work was funded by the Jiangsu Provincial Special Program of Medical Science (BE2017614).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: All patients had written informed consent signed by themselves or their family members prior to their participation in this study. The study was approved by the local ethics committee of the Nanjing Medical University.
References
- Hankey GJ. Stroke. Lancet 2017;389:641-54. [Crossref] [PubMed]
- Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, Moran AE, Sacco RL, Anderson L, Truelsen T, O'Donnell M, Venketasubramanian N, Barker-Collo S, Lawes CM, Wang W, Shinohara Y, Witt E, Ezzati M, Naghavi M, Murray C. Global Burden of Diseases I, Risk Factors S, the GBDSEG. Global and regional burden of stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet 2014;383:245-54. [Crossref] [PubMed]
- Souza LC, Yoo AJ, Chaudhry ZA, Payabvash S, Kemmling A, Schaefer PW, Hirsch JA, Furie KL, Gonzalez RG, Nogueira RG, Lev MH. Malignant CTA collateral profile is highly specific for large admission DWI infarct core and poor outcome in acute stroke. AJNR Am J Neuroradiol 2012;33:1331-6. [Crossref] [PubMed]
- Jung S, Wiest R, Gralla J, McKinley R, Mattle H, Liebeskind D. Relevance of the cerebral collateral circulation in ischaemic stroke: time is brain, but collaterals set the pace. Swiss Med Wkly 2017;147:w14538. [PubMed]
- Paciaroni M, Bandini F, Agnelli G, Tsivgoulis G, Yaghi S, Furie KL, Tadi P, Becattini C, Zedde M, Abdul-Rahim AH, Lees KR, Alberti A, Venti M, Acciarresi M, D'Amore C, Mosconi MG, Cimini LA, Altavilla R, Volpi G, Bovi P, Carletti M, Rigatelli A, Cappellari M, Putaala J, Tomppo L, Tatlisumak T, Marcheselli S, Pezzini A, Poli L, Padovani A, Masotti L, Vannucchi V, Sohn SI, Lorenzini G, Tassi R, Guideri F, Acampa M, Martini G, Ntaios G, Athanasakis G, Makaritsis K, Karagkiozi E, Vadikolias K, Liantinioti C, Chondrogianni M, Mumoli N, Consoli D, Galati F, Sacco S, Carolei A, Tiseo C, Corea F, Ageno W, Bellesini M, Colombo G, Silvestrelli G, Ciccone A, Lanari A, Scoditti U, Denti L, Mancuso M, Maccarrone M, Ulivi L, Orlandi G, Giannini N, Gialdini G, Tassinari T, De Lodovici ML, Bono G, Rueckert C, Baldi A, D'Anna S, Toni D, Letteri F, Giuntini M, Lotti EM, Flomin Y, Pieroni A, Kargiotis O, Karapanayiotides T, Monaco S, Maimone Baronello M, Csiba L, Szabo L, Chiti A, Giorli E, Del Sette M, Imberti D, Zabzuni D, Doronin B, Volodina V, Michel P, Vanacker P, Barlinn K, Pallesen LP, Barlinn J, Deleu D, Melikyan G, Ibrahim F, Akhtar N, Gourbali V, Caso V. Hemorrhagic Transformation in Patients With Acute Ischemic Stroke and Atrial Fibrillation: Time to Initiation of Oral Anticoagulant Therapy and Outcomes. J Am Heart Assoc 2018;7:e010133. [Crossref] [PubMed]
- Thorpe ER, Garrett KB, Smith AM, Reneker JC, Phillips RS. Outcome Measure Scores Predict Discharge Destination in Patients With Acute and Subacute Stroke: A Systematic Review and Series of Meta-analyses. J Neurol Phys Ther 2018;42:2-11. [Crossref] [PubMed]
- Sabarudin A, Subramaniam C, Sun Z. Cerebral CT angiography and CT perfusion in acute stroke detection: a systematic review of diagnostic value. Quantitative imaging in medicine and surgery 2014;4:282. [PubMed]
- Lansberg MG, Straka M, Kemp S, Mlynash M, Wechsler LR, Jovin TG, Wilder MJ, Lutsep HL, Czartoski TJ, Bernstein RA, Chang CW, Warach S, Fazekas F, Inoue M, Tipirneni A, Hamilton SA, Zaharchuk G, Marks MP, Bammer R, Albers GW. investigators Ds. MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): a prospective cohort study. Lancet Neurol 2012;11:860-7. [Crossref] [PubMed]
- Mlynash M, Lansberg MG, De Silva DA, Lee J, Christensen S, Straka M, Campbell BC, Bammer R, Olivot JM, Desmond P, Donnan GA, Davis SM, Albers GW. Investigators D-E. Refining the definition of the malignant profile: insights from the DEFUSE-EPITHET pooled data set. Stroke 2011;42:1270-5. [Crossref] [PubMed]
- Merino JG, Latour LL, Todd JW, Luby M, Schellinger PD, Kang DW, Warach S. Lesion volume change after treatment with tissue plasminogen activator can discriminate clinical responders from nonresponders. Stroke 2007;38:2919-23. [Crossref] [PubMed]
- Jiang L, Peng M, Geng W, Chen H, Su H, Zhao B, Chen YC, Yin X. FLAIR hyperintensities-DWI mismatch in acute stroke: associations with DWI volume and functional outcome. Brain Imaging Behav 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Saver JL, Johnston KC, Homer D, Wityk R, Koroshetz W, Truskowski LL, Haley EC. Infarct volume as a surrogate or auxiliary outcome measure in ischemic stroke clinical trials. The RANTTAS Investigators. Stroke 1999;30:293-8. [Crossref] [PubMed]
- Zheng MZ, Yang QY, Lu XD, Hu SL, Chai C, Shen W, Chang BG, Wang ZY, Xia S. Middle cerebral artery thrombus susceptibility-weighted imaging mapping predicts prognosis. Quant Imaging Med Surg 2019;9:1556-65. [Crossref] [PubMed]
- Azizyan A, Sanossian N, Mogensen MA, Liebeskind DS. Fluid-attenuated inversion recovery vascular hyperintensities: an important imaging marker for cerebrovascular disease. AJNR Am J Neuroradiol 2011;32:1771-5. [Crossref] [PubMed]
- Burke JF, Kerber KA, Iwashyna TJ, Morgenstern LB. Wide variation and rising utilization of stroke magnetic resonance imaging: data from 11 states. Ann Neurol 2012;71:179-85. [Crossref] [PubMed]
- Chen CY, Li CW, Mak HKF, Lin MF, Chan WP. Combined native magnetic resonance angiography, flow-quantifying, and perfusion-imaging for impending second-stroke assessment. Quant Imaging Med Surg 2019;9:521-9. [Crossref] [PubMed]
- Shih YH, Chou HL, Peng YH. Microbial degradation of 4-monobrominated diphenyl ether with anaerobic sludge. J Hazard Mater 2012;213-214:341-6. [Crossref] [PubMed]
- Simonsen CZ, Madsen MH, Schmitz ML, Mikkelsen IK, Fisher M, Andersen G. Sensitivity of diffusion- and perfusion-weighted imaging for diagnosing acute ischemic stroke is 97.5%. Stroke 2015;46:98-101. [Crossref] [PubMed]
- Payabvash S, Taleb S, Benson JC, Rykken JB, Oswood MC, McKinney AM, Hoffman B. The Effects of DWI‐Infarct Lesion Volume on DWI‐FLAIR Mismatch: Is There a Need for Size Stratification? Journal of Neuroimaging 2017;27:392-6. [Crossref] [PubMed]
- Jiang L, Chen YC, Zhang H, Peng M, Chen H, Geng W, Xu Q, Yin X, Ma Y. FLAIR vascular hyperintensity in acute stroke is associated with collateralization and functional outcome. Eur Radiol 2019;29:4879-88. [Crossref] [PubMed]
- Maeda M, Koshimoto Y, Uematsu H, Yamada H, Kimura H, Kawamura Y, Itoh H, Sakuma H, Takeda K. Time course of arterial hyperintensity with fast fluid-attenuated inversion-recovery imaging in acute and subacute middle cerebral arterial infarction. J Magn Reson Imaging 2001;13:987-90. [Crossref] [PubMed]
- Toyoda K, Ida M, Fukuda K. Fluid-attenuated inversion recovery intraarterial signal: an early sign of hyperacute cerebral ischemia. AJNR Am J Neuroradiol 2001;22:1021-9. [PubMed]
- Legrand L, Tisserand M, Turc G, Edjlali M, Calvet D, Trystram D, Roca P, Naggara O, Mas JL, Meder JF, Baron JC, Oppenheim C. Fluid-Attenuated Inversion Recovery Vascular Hyperintensities-Diffusion-Weighted Imaging Mismatch Identifies Acute Stroke Patients Most Likely to Benefit From Recanalization. Stroke 2016;47:424-7. [Crossref] [PubMed]
- McCoy CE, Langdorf MI, Lotfipour S. American Heart Association/American Stroke Association Deletes Sections from 2018 Stroke Guidelines. West J Emerg Med 2018;19:947-51. [Crossref] [PubMed]
- Cocho D, Yarleque S, Boltes A, Espinosa J, Ciurans J, Pont-Sunyer C, Pons J. Clinical Outcome of Ischemic Stroke in Old Patients Versus Oldest-Old. J Stroke Cerebrovasc Dis 2018;27:3657-61. [Crossref] [PubMed]
- Legrand L, Tisserand M, Turc G, Naggara O, Edjlali M, Mellerio C, Mas JL, Meder JF, Baron JC, Oppenheim C. Do FLAIR vascular hyperintensities beyond the DWI lesion represent the ischemic penumbra? AJNR Am J Neuroradiol 2015;36:269-74. [Crossref] [PubMed]
- Higashida RT, Furlan AJ, Roberts H, Tomsick T, Connors B, Barr J, Dillon W, Warach S, Broderick J, Tilley B, Sacks D. Technology Assessment Committee of the American Society of I, Therapeutic N, Technology Assessment Committee of the Society of Interventional R. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke 2003;34:e109-37. [Crossref] [PubMed]
- Singer OC, Berkefeld J, Nolte CH, Bohner G, Reich A, Wiesmann M, Groeschel K, Boor S, Neumann-Haefelin T, Hofmann E, Stoll A, Bormann A, Liebeskind DS. Collateral vessels in proximal middle cerebral artery occlusion: the ENDOSTROKE study. Radiology 2015;274:851-8. [Crossref] [PubMed]
- Karadeli HH, Giurgiutiu DV, Cloonan L, Fitzpatrick K, Kanakis A, Ozcan ME, Schwamm LH, Rost NS. FLAIR vascular hyperintensity is a surrogate of collateral flow and leukoaraiosis in patients with acute stroke due to proximal artery occlusion. J Neuroimaging 2016;26:219-23. [Crossref] [PubMed]
- Mahdjoub E, Turc G, Legrand L, Benzakoun J, Edjlali M, Seners P, Charron S, Ben Hassen W, Naggara O, Meder JF, Mas JL, Baron JC, Oppenheim C. Do Fluid-Attenuated Inversion Recovery Vascular Hyperintensities Represent Good Collaterals before Reperfusion Therapy? AJNR Am J Neuroradiol 2018;39:77-83. [Crossref] [PubMed]
- Nam KW, Kim CK, Kim TJ, Oh K, Han MK, Ko SB, Yoon BW. FLAIR vascular hyperintensities predict early ischemic recurrence in TIA. Neurology 2018;90:e738-44. [Crossref] [PubMed]
- Kim SE, Lee BI, Kim SE, Shin KJ, Park J, Park KM, Kim HC, Lee J, Baek HJ, Jin SC, Ha SY. Clinical Significance of Fluid-Attenuated Inversion Recovery Vascular Hyperintensities in Borderzone Infarcts. Stroke 2016;47:1548-54. [Crossref] [PubMed]
- Shang WJ, Chen HB, Shu LM, Liao HQ, Huang XY, Xiao S, Hong H. The Association between FLAIR Vascular Hyperintensity and Stroke Outcome Varies with Time from Onset. AJNR Am J Neuroradiol 2019;40:1317-22. [Crossref] [PubMed]
- Dong X, Bai C, Nao J. Influential factors and clinical significance of fluid-attenuated inversion recovery vascular hyperintensities in transient ischemic attacks of carotid arterial system. Neuroradiology 2017;59:1093-9. [Crossref] [PubMed]
- Miller MA. Social, economic, and political forces affecting the future of occupational health nursing. AAOHN J 1989;37:361-6. [Crossref] [PubMed]
- Huang X, Liu W, Zhu W, Ni G, Sun W, Ma M, Zhou Z, Wang Q, Xu G, Liu X. Distal hyperintense vessels on FLAIR: a prognostic indicator of acute ischemic stroke. Eur Neurol 2012;68:214-20. [Crossref] [PubMed]
- Siegler JE, Boehme AK, Kumar AD, Gillette MA, Albright KC, Beasley TM, Martin-Schild S. Identification of modifiable and nonmodifiable risk factors for neurologic deterioration after acute ischemic stroke. J Stroke Cerebrovasc Dis 2013;22:e207-13. [Crossref] [PubMed]
- Bang OY, Goyal M, Liebeskind DS. Collateral Circulation in Ischemic Stroke: Assessment Tools and Therapeutic Strategies. Stroke 2015;46:3302-9. [Crossref] [PubMed]
- McVerry F, Liebeskind DS, Muir KW. Systematic review of methods for assessing leptomeningeal collateral flow. AJNR Am J Neuroradiol 2012;33:576-82. [Crossref] [PubMed]
- Lee KY, Latour LL, Luby M, Hsia AW, Merino JG, Warach S. Distal hyperintense vessels on FLAIR: an MRI marker for collateral circulation in acute stroke? Neurology 2009;72:1134-9. [Crossref] [PubMed]
- Hohenhaus M, Schmidt WU, Brunecker P, Xu C, Hotter B, Rozanski M, Fiebach JB, Jungehulsing GJ. FLAIR vascular hyperintensities in acute ICA and MCA infarction: a marker for mismatch and stroke severity? Cerebrovasc Dis 2012;34:63-9. [Crossref] [PubMed]
- Yoo AJ, Verduzco LA, Schaefer PW, Hirsch JA, Rabinov JD, Gonzalez RG. MRI-based selection for intra-arterial stroke therapy: value of pretreatment diffusion-weighted imaging lesion volume in selecting patients with acute stroke who will benefit from early recanalization. Stroke 2009;40:2046-54. [Crossref] [PubMed]
- Parsons MW, Christensen S, McElduff P, Levi CR, Butcher KS, De Silva DA, Ebinger M, Barber PA, Bladin C, Donnan GA, Davis SM. Echoplanar Imaging Thrombolytic Evaluation Trial I. Pretreatment diffusion- and perfusion-MR lesion volumes have a crucial influence on clinical response to stroke thrombolysis. J Cereb Blood Flow Metab 2010;30:1214-25. [Crossref] [PubMed]
- Brito-Filho SB, Moura EG, Santos OJ, Sauaia-Filho EN, Amorim E, Santana EE, Barros-Filho AK, Santos RA. Effect of Chronic Ingestion of Wine on the Glycemic, Lipid and Body Weight Homeostasis in Mice. Arq Bras Cir Dig 2016;29:146-50. [Crossref] [PubMed]
- Timpone VM, Lev MH, Kamalian S, Morais LT, Franceschi AM, Souza L, Schaefer PW. Percentage insula ribbon infarction of >50% identifies patients likely to have poor clinical outcome despite small DWI infarct volume. AJNR Am J Neuroradiol 2015;36:40-5. [Crossref] [PubMed]
- Yoo AJ, Barak ER, Copen WA, Kamalian S, Gharai LR, Pervez MA, Schwamm LH, Gonzalez RG, Schaefer PW. Combining acute diffusion-weighted imaging and mean transmit time lesion volumes with National Institutes of Health Stroke Scale Score improves the prediction of acute stroke outcome. Stroke 2010;41:1728-35. [Crossref] [PubMed]