Percutaneous mechanical atherothrombectomy using the Rotarex®S device in peripheral artery in-stent restenosis or occlusion: a French retrospective multicenter study on 128 patients
Introduction
In-stent restenosis (ISR) of the lower limb arteries is a significant issue that occurs in up to 40% to 50% of cases after percutaneous transluminal angioplasty (PTA) or stenting. It frequently occurs following treatment of long, complex and diffuse lesions. According to the literature, in-stent thrombosis may occur in up to 4.3% of patients, and in a half of these cases it is a consequence of late or very late thrombosis (1). Chronic total re-occlusions are localized, most often in the popliteal artery (PA) and superficial femoral artery (SFA) (1,2). Because of the difficulty with ongoing management, ISR after peripheral angioplasty constitutes a growing therapeutic challenge (2). The incidence of ISR generally ranges from 15–42% at one year (but is documented at up to 73%) (3). Bare metal stents showed a higher rate of ISR and re-occlusion in patients with peripheral arterial disease (PAD). This has led to the development of new stents, including drug-coated stents (DCS). After the DCS, drug-coated balloon (DCB) angioplasty was used as a strategic treatment to reduce ISR in patients with SFA and PA lesions. However, one study showed that approximately 30% of patients treated with DCB angioplasty required an additional stent (4). There is currently no widely-accepted standard treatment for ISR. Percutaneous mechanical debulking (PMD) treatment, has been attempted to lessen restenotic tissue burden and improve patency while reducing target lesion revascularization (TLR). The debulking of the restenotic stent may potentially improve DCB treatment effects by reducing the thickness of thrombus and hyperplastic tissue and increasing drug dosage transit to the intima. However, only few studies have reported results of debulking devices in such a setting. Furthermore, all were monocentric including small number of patients.
In this context, we therefore undertook a retrospective multicentre study to ascertain the safety and mid-term outcomes of Rotarex®S rotational atherectomy plus thrombectomy (Straub Medical AG, Wangs, Switzerland) with or without adjunctive treatment (PTA/DCB/stenting) in patients with ISR or reocclusion in the iliac and/or infrainguinal arteries.
Methods
From January 15, 2013, till November 19, 2018, we retrospectively analysed 128 treatments for lower limb ISR by PMD.
Patient cohort
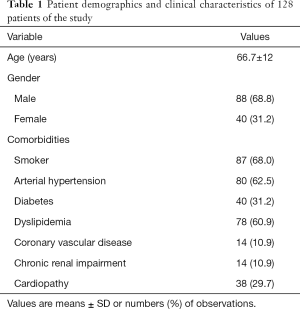
There were 128 symptomatic patients (88 men and 40 women) with ISR thrombosis of a previously implanted stent who underwent PMD treatment. The mean age was 66.7±12 years (median, 65 years) ranging from 39 to 94 years. Risk factors and co-morbidities of patients such as high blood pressure, diabetes, smoking, dyslipidemia, heart disease and chronic renal failure were collected (Table 1). Patients with an acute (<14 days), subacute (<3 months) or chronic (>3 months) onset of ischemia were included who showed either a stenosis (50 events, 39.1%) or an occlusion (76 events, 59.4%) in-stent lesion. They were categorized according to the Rutherford-Becker classification (RBC). Patients presented with limb ischemia Rutherford category II–III (moderate to severe claudication) in 62 cases (48.4%), Rutherford category IV (ischemic rest pain) in 44 instances (34.4%) and Rutherford V–VI (minor tissue loss non-healing ulcer, focal gangrene with diffuse pedal ischemia to major tissue loss-extending above transmetatarsal level, functional foot no longer salvageable) in 22 cases (17.2%). Thus the cohort included 51.5% CLI patients. The main inclusion endpoints were acute, subacute or chronic ISR (>50%) or CTO, Rutherford category 2 to 6 in the target limb, iliac and/or infrainguinal arteries and successful intraluminal guidewire crossing of target lesions. The exclusion criteria were below-the-knee (BTK) lesions.
Due to the retrospective nature of this study, our Ethics Committee waived the requirement for informed patient consent.
Full table
Lesion characteristics and procedural details
Patients were classified by the location of their occlusion (Table 2) as iliac arteries (IA), SFA and PA. The PMD device used was the Rotarex®S System (Straub Medical, Wangs, Switzerland), with 6-, 8- or 10-French (Fr) sheath compatible devices, depending on the vessel diameter to be treated. The length of the lesions was visually estimated on digital subtraction angiography.
Full table
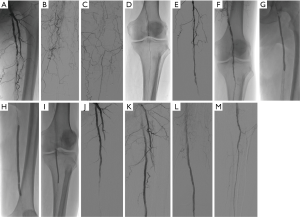
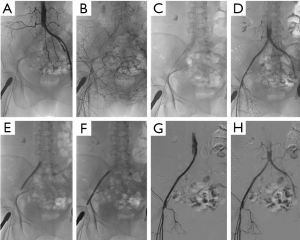
The approaches performed during the interventions included common femoral ipsilateral, common femoral contralateral or brachial access. The Rotarex®S device was inserted over a 0.018-inch guidewire until a few centimetres proximal to the lesion. Several passes were necessary to eliminate the thrombus. In case of a persistent underlying severe stenosis or residual prominent thrombus, certain adjunctive treatments such as the PTA, DCB or both were utilised (Figures 1,2). Mean runtime was 5.1±2.3 minutes with the Rotarex®S device. A stent was implanted if residual stenosis diameter was >50%. The new stent was positioned either in the same place as the previous one, or at another location or both. An appropriate closure device was used to obtain hemostasis in case of complications. Post-interventional medication included anticoagulation therapy as a curative or preventive measure. After the procedure, an antiplatelet therapy single or dual (aspirin 150 mg/day and clopidogrel 75 mg/day) was recommended for at least 12 weeks to avoid thrombotic events.
Study device
The catheter head is made up of two overlying metal cylinders, with two side openings. The outer cylinder is connected to the rotating helix, and the inner cylinder to the catheter shaft. The helix and the catheter head rotate at approximately 40,000–60,000 rpm depending on the model, by means of a gear box in the catheter housing and a motor contained within the catheter handle driven by the Drive System. The rotating outer cylinder is fitted with facets at its foremost tip which when rotating, serve to abrade occluding material lying in front of it.
When in operation, both the helix and the outer catheter head, rotate and are advanced along the guidewire toward an arterial occlusion. When an occlusion is met, the rotating head, with its small, blunt facets in its forward aspect, breaks down the occlusive material. Concomitantly, the rotation of the catheter head creates a vortex in the blood which assists to further erode occluding material from the vessel lumen. The rotating helix produces a negative pressure inside the catheter tube and acts as a conveyor screw upon which the ablated material is transported. The detached particles are drawn into the catheter through side windows in the head where they are further broken down and drawn out of the body and into the attached collecting bag under continuous aspiration. At no time is it necessary for the catheter or rotating head to come into contact with the vessel wall in order to be effective. The catheter is designed in such a manner that when used as directed, over a guidewire and with adequate proximal blood flow, no wall damage would result if contact with a vessel wall should unintentionally occur. There are different catheter sizes: 6-, 8- and 10-Fr.
Three lengths are useful depending on the size of the lesion: 85, 110 and 135 cm. They are free of phthalates and latex. They are composed of three parts: cutting-head, helix and side window, respectively (Figure 3). The suction performance is about 0.66 mL/s with the 6-Fr system and 1.5 mL/s with the 8-Fr system (5).
Data collection
Success
Technical success was defined as successful completion of the procedure and recanalization of the entire occlusion length with ≤30% diameter residual stenosis.
Clinical success for patients with claudication (Rutherford 2) was defined as clinical improvement of at least one clinical category and for patients with rest pain (Rutherford 3) or CLI (Rutherford 4-5-6) was defined as the resolution of ischemic rest pain and the healing of ischemic ulcers.
TLR was defined as any repeat percutaneous intervention of the target lesion or bypass surgery of the target vessel performed for restenosis or other complication of the target lesion.
Follow-up and endpoints
All patients were routinely scheduled to return for ambulatory follow-up visits at 30 days and then at 3, 6 and 12 months. The key endpoints included safety, technical success, clinical success, patency rates of the in-stent lesion, limb salvage and survival at 1, 3, 6 and 12 months. Other outcome measures are, rate of major adverse events (MAE) defined as death, myocardial infarction (MI), stroke, renal failure and major complications (requiring hospitalization and/or reintervention) including dissection, perforation, bleeding, thrombosis, embolization, false aneurysm and infection between 30-day and 12-month follow-up period.
Statistical analysis
Categorical variables are summarized as percentages and proportions and compared using the Chi-square test or Fisher exact test, as appropriate. Continuous variables are presented as mean ± standard deviation or as median with range. Kaplan-Meier survival analysis and the log-rank test were performed to assess primary and secondary patency rates after different procedures. Statistical significance was defined as P<0.05.
Results
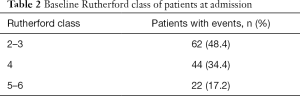
A total of 128 patients (88 men and 40 women) were included in this study. Table 1 highlights patient demographics and clinical characteristics as well as cardiovascular risks factors. Over 50% of our patients had a Rutherford score of 4–6 (i.e., CLI). Table 2 shows lesions Rutherford classification.
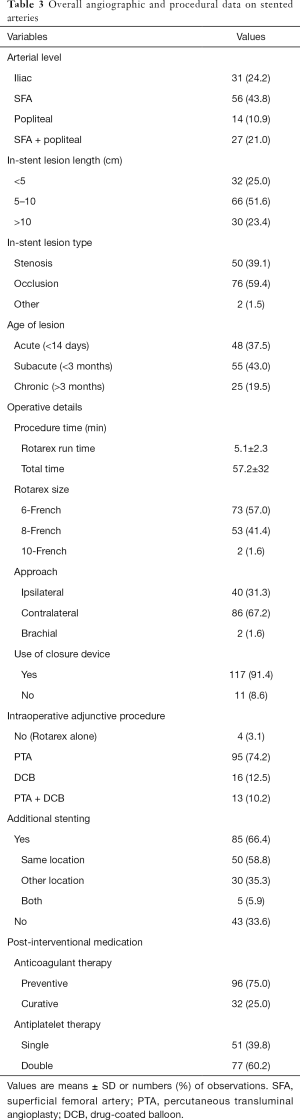
In the majority of patients (n=66; 51.6%), the in-stent lesion length was between 5 and 10 cm with SFA alone (n=56; 43.8%) and iliac (n=31; 24.2%) vessels segments as the mostly treated. The majority of patients presented with an in-stent occlusion (n=76; 59.4%) and the age of lesion observed were acute or subacute lesion. The median time of the interventional procedure was 57.2±32 min but the runtime of Rotarex®S lasted a mere 5.1±2.3 min. We used Rotarex®S device alone only in 4 (3.1%) cases. Others cases all have an adjunctive treatment (PTA, DCB or PTA + DCB). In 75% of the patients, we used post-interventional medication preventatively. A contralateral approach was used in more than 80 interventions, i.e., 67.2% of the time. During the intervention, a stent-in-stent was performed in 85 patients, either in the same or another location. Operative details and overall angiographic data are outlined in Table 3.
Full table
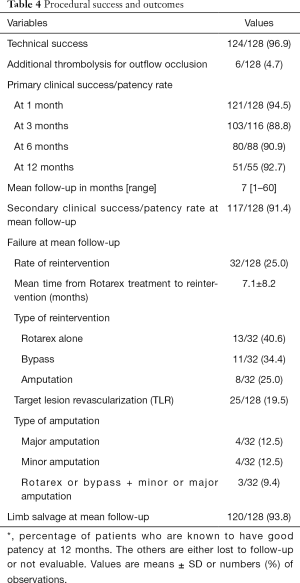
In 124 of 128 cases, a primary technical success (residual stenosis ≤50%) of 96.9% was obtained with a complete recanalization of the previous stented lesions. At one-month follow-up, clinical success was of 94.5% and at 12 months 92.3% of the evaluable population. At a mean follow-up of 7 months (range of 1–60), the secondary reintervention rate was 25% (32 patients). Among them, 40.6% (13 patients) were treated by Rotarex®S alone or 34.4% (11 patients) with bypass and 25% (8 patients) have undergone an amputation. TLR was 19.5% (25 patients). Limb salvage at mean follow-up was 93.8%.
Table 4 shows procedure success of the study. The overall primary clinical success rate at three-month follow-up was 88.8%. Primary patency/clinical success at 30 days was 94.5%. At mean follow-up, 90% of patients evaluable (Figure 4). Kaplan-Meier curve (Figure 5) showed that freedom from any reintervention was over 50% at 41.5 months.
Full table
At one-month follow-up, there were acute complications requiring hospitalization and/or reintervention. Among other things, we observed 3 perforations (2.3%), 1 device defect (0.8%) and 7 embolizations (5.5%). One (0.8%) patient died and 1 (0.8%) patient had suffered a major amputation. Detailed data on major complications and MAE during follow-up are described in Table 5. Overall MAE, at mean follow-up showed a mortality rate of 14.1%. Others MAE are outlined in Table 6.
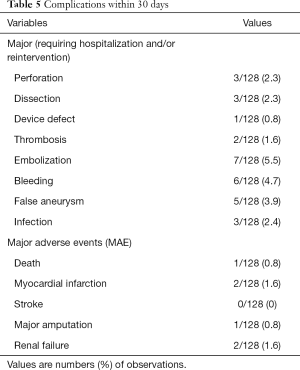
Full table
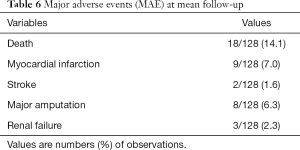
Full table
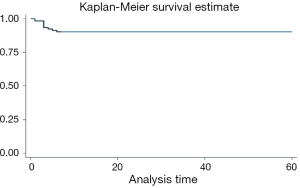
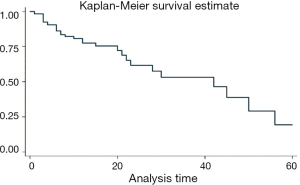
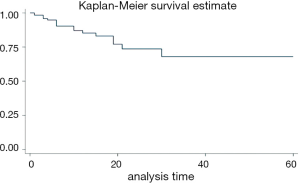
Kaplan-Meier curve (Figure 6) showed a survival rate of 68% at 60 months. In our study, 32 reinterventions were performed. Of these 32 reinterventions, 10 (13%) of 77 patients had double antiplatelet therapy. The other 22 (43.1%) were on single antiplatelet therapy. Reintervention rate was lower in patients under antiplatelet therapy vs. single antiplatelet therapy (P<0.05). There was no impact of lesion type (length, degree, age) on patency/reintervention/survival rates (P>0.05).
Discussion
In recent years, ISR following peripheral angioplasty or stenting has become a major therapeutic challenge driving the development of new innovative medical devices. Recent studies and case reports have shown the high technical success rates of Rotarex®S in the treatment of stenosis and occlusion of native peripheral arteries (6-8). However, few studies have evaluated the success of this safe and effective technique in the treatment of ISR in the lower limbs.
This article presents a 5-year study whose main objective was to evaluate the technical and clinical success of revascularization of the stented peripheral arteries with PMD by the Rotarex®S medical device. In our study this treatment was combined with either PTA or with the DCB or both. In our series, we achieved a primary patency rate of 92.3% at 12 months all techniques combined. Secondary clinical success at mean follow-up was 117/128 (91.4%) after reinterventions. The overall reintervention rate observed was 25%. The TLR was 25/128 (19.5%). The dissection rate at 30 days was 2.3% and perforation rate of 2.3%. The risk of vessel perforation or dissection is mainly dependent on the safe, intraluminal position of the guidewire. Calcified arteries were at high risk to be perforated. Moreover, lesion type (length, degree, age) did not influence the patency, reintervention or survival rates (P>0.05). Milnerowicz et al. made the same observation (2).
Several devices and techniques can be used to treat femoropopliteal artery disease. Bare metal stents implantation is commonly used. Stents resolve the problems of elastic recoil, residual stenosis and dissection but ISR stay high with an incidence of 15% to 32% in 12 months (3,9). Dick et al. showed the ISR treatment with angioplasty resulted in a restenosis recurrence rate with up to 73% (10). Cutting balloon angioplasty has proven to be more successful but the restenosis recurrence rate showed it was not statistically significant (65% vs. 73%) (10). Zeller et al. had obtained better results with implantation of paclitaxel-coated stents. The primary patency rate at one year was 78.8% of the lesion with mean lesion length of 133±91.7 mm and occlusion rate of 30% (11).
Table 7 shows the outcomes of several treatment techniques for femoropopliteal ISR. Several studies have documented positive outcomes of hybrid treatment for restenotic lesions combining atherectomy (laser, directional) with DCB angioplasty (2). The EXCITE ISR trial is the first large, randomized and prospective study to demonstrate superiority of laser atherectomy + PTA versus PTA alone. The first group had shown a better procedural success (93.5% vs. 82.7%, P=0.01) with few complications. At six months, the freedom from TLR was 73.5% versus 51.8% (P<0.005) (15).

Full table
The Rotarex®S system is a purely mechanical endovascular atherectomy plus thrombectomy device (17). It was reported by Freitas et al. that 6-Fr Rotarex®S device is used in the treatment of acute mesenteric ischemia or acute limb ischemia with no mortality, few complications and great outcomes (18). The main advantage of this technique is safe, rapid and effective removal of large thrombus volume (19). Kronlage et al. conducted a comparative study of two intervention methods, PMD and local thrombolysis for the treatment of acute and subacute ischemia of limbs in thrombotic occlusions of peripheral limbs. Their results show a primary patency success of more than 98% for both techniques used. At 12 months follow-up, primary and secondary patency after PMD alone was significantly better compared to local thrombolysis or Rotarex®S and lysis (63% and 85%, P<0.05). Overall survival 12 months after surgery was 96% in patients without critical illness and survival without amputation was 94.3%. Direct comparison between the different intervention techniques in this study showed that the rate of major bleeding was significantly lower in patients treated with Rotarex®S than in patients receiving thrombolysis (20). Stanek et al. showed immediate successful recanalization in 69 interventions performed (95%) in 65 patients with acute and subacute occlusion in the peripheral arteries, in an evaluation of the immediate and long-term outcomes of the Rotarex®S catheter. Peripheral embolization was the most common transient complication (6%). Thus, the Rotarex®S system provided fast and effective treatment of peripheral arterial thromboembolic occlusions. It is a safe tool for the treatment of in-stent acute and subacute arterial thromboembolic occlusions or even chronic peripheral thromboembolic occlusions. According to the authors, it can be used for short or long occlusions with the same success, provided that the obstruction is not strongly calcified and that a guidewire has been safely passed through the obstruction (21). It has been shown by other authors too (22,23).
Our study included 128 patients with ISR in femoropopliteal arteries and iliac arteries with cardiovascular co-morbidities. It is the largest multicentre series published to date despite limitations. First, this was a non-randomized, retrospective study with a modestly-sized cohort with absence of control group. Furthermore, we had heterogeneous population with different target vessels (iliac vs. SFA vs. PA vs. combined), different stent occlusions like restenosis vs. occlusion, acute thrombosis vs. chronic thrombosis vs combined and the use of different techniques (debulking alone vs. debulking + PTA vs. debulking + DCB vs. combined). The outcomes need to be confirmed in a larger study or randomized controlled trial with longer follow-up.
In conclusion, this study showed that Rotarex®S rotational debulking device alone or associated with PTA or/and DCB angioplasty is safe, quick and effective in patients with in-stent occlusion or restenosis in iliac and infrainguinal arteries. Mechanical thrombectomy and atherectomy are efficient methods of arterial debulking to achieve recanalization when used in the treatment of acute, subacute or even chronic occlusion or stenosis of peripheral arteries. It is also safe when performed by less experienced operators. It may produce satisfactory outcomes in patients with in-stent occlusion in peripheral arteries in terms of primary patency rate. This is the largest and only multicentre study that has analysed the outcomes of such a hybrid intervention in the treatment of in-stent occlusion. Its results justify further research in the application of the therapy to determine cost/benefit.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This retrospective study was performed in compliance with the requirements of the institutional review board and approved by the institution ethical committee. Informed consent was waived.
References
- Banerjee S, Sarode K, Mohammad A, Gigliotti O, Baig MS, Tsai S, Shammas NW, Prasad A, Abu-Fadel M, Klein A, Armstrong EJ, Jeon-Slaughter H, Brilakis ES, Bhatt DL. Femoropopliteal artery stent thrombosis: report from the excellence in peripheral artery disease registry. Circ Cardiovasc Interv 2016;9:e002730. [Crossref] [PubMed]
- Milnerowicz A, Milnerowicz A, Kuliczkowski W, Protasiewicz M. Rotational atherectomy plus drug-coated balloon angioplasty for the treatment of total in-stent occlusions in iliac and infrainguinal arteries. J Endovasc Ther 2019;26:316-21. [Crossref] [PubMed]
- Shishehbor MH. Endovascular treatment of femoropopliteal lesions: so many options, little consensus. J Am Coll Cardiol 2015;66:2339-42. [Crossref] [PubMed]
- Scheinert D, Duda S, Zeller T, Krankenberg H, Ricke J, Bosiers M, Tepe G, Naisbitt S, Rosenfield K. The LEVANT I (Lutonix paclitaxel-coated balloon for the prevention of femoropopliteal restenosis) trial for femoropopliteal revascularization: first-in-human randomized trial of low-dose drug-coated balloon versus uncoated balloon angioplasty. JACC Cardiovasc Interv 2014;7:10-9. [Crossref] [PubMed]
- Freitas B, Steiner S, Bausback Y, Branzan D, Ulrich M, Bräunlich S, Schmidt A, Scheinert D. Rotarex mechanical debulking in acute and subacute arterial lesions. Angiology 2017;68:233-41. [Crossref] [PubMed]
- Wissgott C, Kamusella P, Richter A, Klein-Wiegel P, Steinkamp HJ. Mechanical rotational thrombectomy for treatment thrombolysis in acute and subacute occlusion of femoropopliteal arteries: retrospective analysis of the results from 1999 to 2005. Rofo 2008;180:325-31. [Crossref] [PubMed]
- Zeller T, Frank U, Bürgelin K, Schwarzwälder U, Horn B, Flügel PC, Neumann FJ. Long-term results after recanalization of acute and subacute thrombotic occlusions of the infra-aortic arteries and bypass-grafts using a rotational thrombectomy device. Rofo 2002;174:1559-65. [Crossref] [PubMed]
- Lichtenberg M, Stahlhoff FW, Boese D. Endovascular treatment of acute limb ischemia and proximal deep vein thrombosis using rotational thrombectomy: a review of published literature. Cardiovasc Revasc Med 2013;14:343-8. [Crossref] [PubMed]
- Kim W, Choi D. Treatment of femoropopliteal artery in-stent restenosis. Korean Circ J 2018;48:191-7. [Crossref] [PubMed]
- Dick P, Sabeti S, Mlekusch W, Schlager O, Amighi J, Haumer M, Cejna M, Minar E, Schillinger M. Conventional balloon angioplasty versus peripheral cutting balloon angioplasty for treatment of femoropopliteal artery in-stent restenosis: initial experience. Radiology 2008;248:297-302. [Crossref] [PubMed]
- Zeller T, Dake MD, Tepe G, Brechtel K, Noory E, Beschorner U, Kultgen PL, Rastan A. Treatment of femoropopliteal in-stent restenosis with paclitaxel-eluting stents. JACC Cardiovasc Interv 2013;6:274-81. [Crossref] [PubMed]
- Krankenberg H, Tubler T, Ingwersen M, Schluter M, Scheinert D, Blessing E, Sixt S, Kieback A, Beschorner U, Zeller T. Drug-coated balloon versus standard balloon for superficial femoral artery in-stent restenosis: the randomized Femoral Artery In-stent Restenosis (FAIR) trial. Circulation 2015;132:2230-6. [Crossref] [PubMed]
- Kinstner CM, Lammer J, Willfort-Ehringer A, Matzek W, Gschwandtner M, Javor D, Funovics M, Schoder M, Koppensteiner R, Loewe C, Ristl R, Wolf F. Paclitaxel eluting balloon versus standard balloon angioplasty in in-stent restenosis of the superficial femoral and proximal popliteal artery: 1-year results of the PACUBA trial. JACC Cardiovasc Interv 2016;9:1386-92. [Crossref] [PubMed]
- Bague N, Julia P, Sauguet A, Pernès JM, Chatelard P, Garbé JF, Penillon S, Cardon JM, Commeau P, Planché O, Guyomarch B, Gouëffic Y. Femoropopliteal in-stent restenosis repair: midterm outcomes after paclitaxel eluting balloon use (PLAISIR trial). Eur J Vasc Endovasc Surg 2017;53:106-13. [Crossref] [PubMed]
- Dippel EJ, Makam P, Kovach R, George JC, Patlola R, Metzger DC, Mena-Hurtado C, Beasley R, Soukas P, Colon-Hernandez PJ, Stark MA, Walker C. EXCITE ISR Investigators. Randomized controlled study of excimer laser atherectomy for treatment of femoropopliteal in-stent restenosis: initial results from the EXCITE ISR trial (EXCImer Laser Randomized Controlled Study for Treatment of FemoropopliTEal In-Stent Restenosis). JACC Cardiovasc Interv 2015;8:92-101. [Crossref] [PubMed]
- Schmidt A, Zeller T, Sievert H, Krankenberg H, Torsello G, Stark MA, Scheinert D. Photoablation Using the Turbo-Booster and excimer laser for in-stent restenosis treatment: twelve-month results from the PATENT study. J Endovasc Ther 2014;21:52-60. [Crossref] [PubMed]
- Giusca S, Raupp D, Dreyer D, Eisenbach C, Korosoglou G. Successful endovascular treatment in patients with acute thromboembolic ischemia of the lower limb including the crural arteries. World J Cardiol 2018;10:145-52. [Crossref] [PubMed]
- Freitas B, Bausback Y, Schuster J, Ulrich M, Bräunlich S, Schmidt A, Scheinert D. Thrombectomy devices in the treatment of acute mesenteric ischemia: initial single-center experience. Ann Vasc Surg 2018;51:124-31. [Crossref] [PubMed]
- Liu J, Li T, Huang W, Zhao N, Liu H, Zhao H, Wang H. Percutaneous mechanical thrombectomy using Rotarex catheter in peripheral artery occlusion diseases - Experience from a single center. Vascular 2019;27:199-203. [Crossref] [PubMed]
- Kronlage M, Printz I, Vogel BB, Blessing E, Müller OJ, Katus HA, Erbel C. A comparative study on endovascular treatment of (sub)acute critical limb ischemia: mechanical thrombectomy vs thrombolysis. Drug Des Devel Ther 2017;11:1233-41. [Crossref] [PubMed]
- Stanek F, Ouhrabkova R, Prochazka D. Mechanical thrombectomy using the Rotarex catheter in the treatment of acute and subacute occlusions of peripheral arteries: immediate results, long-term follow-up. Int Angiol 2013;32:52-60. [PubMed]
- Silingardi R, Cataldi V, Moratto R, Azzoni I, Veronesi J, Coppi G. Mechanical thrombectomy in in-stent restenosis: preliminary experience at the iliac and femoropoliteal arteries with the Rotarex system. J Cardiovasc Surg (Torino) 2010;51:543-50. [PubMed]
- Liao CJ, Song SH, Li T, Zhang Y, Zhang W. Combination of rotarex thrombectomy and drug-coated balloon for the treatment of femoropopliteal artery in-stent restenosis. Ann Vasc Surg 2019;60:301-7. [Crossref] [PubMed]