
Current concepts for the diagnosis of acute pancreatitis by multiparametric magnetic resonance imaging
Introduction
Acute pancreatitis (AP), a common acute abdominal condition, has a prevalence of up to 50 per 100,000 individuals each year (1-3). It is characterized by acute chemical inflammation in the pancreas resulting from leakage of activated pancreatic digestive enzymes, leading to autodigestion of pancreatic parenchyma and peripancreatic tissues (4-6). The known etiological factors for AP include choledocholithiasis, cholangio-pancreatic duct variants, the intraductal papillary mucinous tumor of the pancreas, along with other alcohol-, hyperlipidemia-, and surgery-related factors (1,5-7). Currently, the clinical varieties of AP are divided into three categories delineated by the 2012 revised Atlanta Classification using clinical findings and contrast-enhanced computerized tomography (CECT) (4). Mild AP (around 40% of patients), exhibits a self-limiting disorder without any organ dysfunction and absence of local complications, along with a favorable prognosis (4-8). Moderately severe AP (around 50% of patients) refers to transient organ dysfunction (resolving within 48 hours) or the presence of local complications, with a relatively lower mortality rate of approximately 2% (4-8). Finally, severe AP (around 10% of patients) is defined by persistent organ dysfunction (persists more than 48 hours) and usually has a higher prevalence of local complications based upon CECT, in addition to a significantly higher mortality rate of 20–30% (4-8). The diagnosis of AP and related severity assessments are primarily based on clinical manifestations and a series of laboratory examinations. Various imaging modalities also play significant roles in readily confirming the diagnosis of AP, helping identify the degree of pancreatic or extrapancreatic necrosis (extra- or peripancreatic necrosis alone, is another important entity associated with acute necrotizing pancreatitis in the 2012 revised Atlanta Classification), staging the severity of AP in the early phase, differentiating AP from other comorbidities associated with acute abdomen, and detecting a variety of early or delayed local complications (4-6).
Although CECT has been considered a traditional first-line cross-sectional imaging modality for AP and for exploring the thorax and pelvis, it is a potential radiation risk when used for multiple follow-up scans (9,10). Compared with CECT, magnetic resonance imaging (MRI) is a reliable diagnostic technique with multi-parameter-imaging, better soft-tissue contrast, and no risk of radiation, which could be valuable for AP patients requiring multiple follow-up examinations. Given these facts, that the 2012 revised Atlanta Classification is primarily based on CT can be considered a major weakness. Furthermore, some local complications, such as a small amount of necrotic/fat debris, are difficult to distinguish accurately on CT within one collection. A non-enhanced MRI is also superior to CECT for confirmation of mild AP (11); meanwhile, MRI in combination with MR cholangiopancreatography (MRCP), a single diagnostic modality, has the advantages of demonstrating noninvasive images of few necrotic/fat materials within a fluid-containing lesion and pancreatic duct systems for duct integrity information (MRCP images), and evaluating whether peripancreatic collections are communicating with pancreatic ducts (12). The increasingly widespread use of new MRI techniques has allowed the prompt recognition of AP-related pathologic conditions, thereby improving the choice of management.
In this article, we review and discuss the diagnostic value that the utility of multiparametric MRI techniques in characterizing and differentiating pathologies in AP patients, including necrosis, omental or mesenterical changes, hemorrhage, and infection. The classic MRI appearances of the most common forms of local complications compared to the 2012 revised Atlanta Classification are also described and illustrated.
MRI protocol
For comprehensive imaging of AP, it is crucial to assess all portions of the pancreatic parenchyma, pancreatic ducts, peripancreatic tissues, and local complications. The multiparametric MRI protocol for the assessment of AP at our institution includes (I) T1-weighted fat-suppressed imaging [single-breath-hold gradient echo (GRE) sequence], (II) T2-weighted fat-suppressed imaging [turbo spin-echo (TSE) or half-Fourier acquisition single-shot turbo spin-echo (HASTE) sequence], (III) diffusion-weighted imaging (DWI) and apparent diffusion coefficient (ADC) map, (IV) two- or three-dimensional MRCP (HASTE heavily T2-weighted sequence), and (V) dynamic contrast-enhanced (DCE) MR imaging [T1-weighted acquisition performed by three-dimensional volumetric interpolated breath-hold examination (3D VIBE)]. The detailed parameters used on our 3.0-T MR scanner (Magnetom Prisma, Siemens Healthcare, Erlangen, Germany) for these sequences are shown in Table 1.

Full table
Contrast material intravenous administration at our institution is performed with 3D fat-saturated VIBE T1-weighted contrast-enhanced-MRI sequencing. It typically takes around 65 s using three-phase dynamic scans (15 s for each phase and 10 s of the interval between phases for breathing). Usually, a dose of 0.1 mmol/kg gadolinium-based contrast agent is used, and a bolus of 20-mL of physiologic saline is immediately administered thereafter. Contrast material and physiologic saline are routinely injected by an infusion rate of 3–4 mL/s with a power injector.
The strengths and limitations of the aforementioned sequences for evaluation of AP are indicated as follows:
- GRE T1-weighted with fat-suppressed images are particularly useful for the delineation of the lace-like contours of the pancreas and pancreatic boundaries, which have important value in defining a focal or diffuse enlarged pancreas (13). In addition, the use of T1-weighted images is useful for the assessment of changes in the peripancreatic fat, a feature of interstitial pancreatitis. The finding mainly includes spotted, patchy, or fat stranding areas of decrease in signal intensity within a surrounding pancreatic bed. T1-weighted imaging is also helpful in showing pancreatic necrosis. Furthermore, this sequence improves evaluation for the presence of hemorrhage according to hyperintensity in the pancreatic and peripancreatic areas during the early and late phases after AP onset (13,14). However, T1-weighted imaging with breath-hold requires high patient cooperation; otherwise, as severe AP patients are frequently out of breath, there can be conspicuous respiratory motion artifacts that can affect the visualization of images.
- TSE or HASTE T2-weighted with fat-suppressed images have a significant advantage in accurately depicting pancreatic interlobular septal abnormalities (inflammation and edema), abnormalities of pancreatic covering (edematous thickening), and fluid collections in and around the pancreas (13,15). Additionally, this sequence is helpful for detecting solid components/necrotic debris within peripancreatic collections (mentioned below). Apart from this, navigator-echo-based sequences or respiratory-triggered approaches have been used for investigating patients less able to comply with imaging procedures.
- SE-EPI DWI can be helpful in AP imaging, especially in the detection of necrosis or hemorrhage and in characterizing infection complications. Some studies have found DWI to be useful in the identification of AP with greater diffusion restriction and lower ADC value in infectious collections, as compared with healthy control individuals (16,17). However, there is potential overlap of ADCs between different pathological processes of AP.
- HASTE MRCP image has the valuable ability of noninvasively assessing the main pancreatic duct, side-branches, and entire extra-hepatic biliary tracts in the late phase of AP (15,17). Three-dimensional MRCP, in particular, can improve the visualization of main pancreatic duct leakage or disruption (18,19). However, the major limitation of MRCP is decreased ductal visibility resulting from the overlap of adjacent fluid-containing organs or structures (e.g., the stomach, duodenum, kidney, and spinal canal) and edematous mesentery in the acute period of AP. Moreover, although secretin-MRCP can be considered a noninvasive modality that can depict the whole parenchyma and pancreatic ducts, due to ductal disruption and ductal leak, many institutions view AP as a direct contraindication to secretin administration. Indeed, previous reports (19) have advised against administering secretin within 3 weeks of the onset of an acute episode of AP.
- 3D VIBE CE-MRI images are used to evaluate the blood supply of the pancreas, and the presence and extent of necrosis of the pancreas in the early phase of AP, which have been shown to correlate to the severity of AP with CECT (8,9). In addition, CE-MRI provides better information for the visualization of peripancreatic vascular involvements like pseudoaneurysm or phlebothrombosis (13,15). However, the primary drawbacks of CE-MRI comprise necessitating intravenous gadolinium-based agent administration and thus incurring a potential risk of interstitial fibrosis (20).
- Compared with CT, the main weakness of MRI for AP is the lack of systematic study of the thorax and the pelvis. However, it is usually not necessary to systematically exam them, as an abdomen baseline status is more associated with the severity of this disease.
MRI characteristics
Pancreatic and/or peripancreatic necrosis
Based on pathological changes, AP is divided into two groups: acute interstitial edematous pancreatitis and acute necrotizing pancreatitis. From this, acute necrotizing pancreatitis can be further stratified into three subcategories according to the 2012 revised Atlanta Classification: (I) combined pancreatic and peripancreatic necrosis (nearly 75%), (II) peripancreatic necrosis only (nearly 20%), and (III) pancreatic necrosis only (less than 5%) (4-7). Classically, pancreatic necrosis (PN), a pivotal imaging marker of necrotizing pancreatitis, is defined as focal, multifocal or diffuse, or superficial or deep devitalized tissue in the pancreatic gland (13,15). Usually, PN merges and matures definitively between 3 and 5 days from symptom onset, as identified by imaging for necrotizing pancreatitis (4-7). Importantly, recognition of PN by means of clinical judgment is unreliable; thus, the use of diagnostic imaging for this purpose has been emphasized. Furthermore, intravenous administration of contrast agent is often essential to improve the differentiation of real necrosis from transient pancreatic ischemia or edema alone (5-8). On MR images, necrotic tissues can be accurately diagnosed when typically spotted, patchy, or with large hypointense areas on fat-suppressed T1WI/T2WI (although they may be hyperintense if liquefied), and if distinct areas of non-enhanced glandular tissue, opposed to intensely enhanced parenchyma of normal pancreatic portion on CE-MRI, are present (Figure 1). According to the amount of PN, its percentage at CE-MRI can be further identified as less than 30% (mild), 30–50% (moderate), and more than 50% (severe) of the pancreatic gland. The sequential MR severity index (MRSI), which is similar to the CT severity index (CTSI) (21,22), can also be recorded. Indeed, patients with PN, particularly those with extensive necrosis (> 30%), should routinely be monitored in the ICU, as there is a well-established increase of mortality correlated directly to the presence and extent of PN (23). In addition, the clinical significance of the MRSI may predict the severity of AP with initial MRI images during the early phase of AP, and the MRSI is significantly correlated to the APACHE II score, the systemic complication occurrence rate, hospitalization stay, and clinical outcome (24-26).
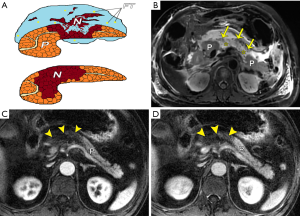
Recently, with a growing understanding of necrosis, extra- or peripancreatic necrosis alone (EPN or PPN, respectively) has been considered as another important entity associated with acute necrotizing pancreatitis. EPN/PPN solely accounts for approximately 20% of cases with acute necrotizing pancreatitis (4-8). According to the 2012 revised Atlanta Classification, the clinical severity of patients with EPN or PPN alone is defined as the severity between patients with edematous pancreatitis and those with pancreatic parenchyma necrosis (4-8). The prognosis of patients with EPN or PPN alone is usually better than that of patients with pancreatic parenchyma necrosis, but not as good as cases with interstitial edematous pancreatitis (8,27). Thus, it is important for radiologists in clinical practice to make a correct, early diagnosis and to differentiate pure EPN or PPN from combined parenchyma necrosis. In fact, EPN or PPN is predominantly associated with necrosis of retroperitoneal and/or mesenterical and omental fatty tissues (discussed below). On MRI, fat-suppressed sequences, when extrapancreatic changes exceed fat stranding, especially spotted or patchy areas of confluent decrease in signal intensity within surrounding collections, and this is combined with a lack of non-enhanced pancreatic parenchyma, EPN or PPN alone may be diagnosed (Figure 2). However, one weakness of MRI is its small field of view (large areas of the chest and pelvic cavity cannot be obtained at one time as CT does), and EPN may be far from the pancreatic area (e.g., in the pelvis or thorax).
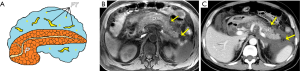
Retroperitoneal, mesenterical, and omental changes/fat necrosis
Extravasation of activated pancreatic enzymes following an episode of AP can result in the occurrence of retroperitoneal, mesenterical, and omental fat edema combined with a varying extent of necrosis. This is a common phenomenon that takes place in cases with or without PN (13,15,27). Previous studies demonstrated the capacity of CT to diagnose the retroperitoneal fat necrosis is limited (21,22). MRI can be used effectively to visualize the intra-abdominal inflammatory spread involving mesenteric and omental fatty regions (Figure 3), as a pathological condition of intra-abdominal fat edema admixed with fat necrosis secondary to AP. We studied the MRI features of mesocolon involvement in AP and the correlation between mesocolon involvement and the severity of AP (28). We found that 61.9% had transverse-mesocolon involvement (TMI) among our 210 AP patients. In addition, depending on the TMI grading, TMI was significantly associated with MRSI scores (r = 0.759, P <0.001) and acute physiology and chronic health evaluation (APACHE) II scores (r = 0.384, P <0.001), supporting TMI’s use as a potential supplementary indicator for AP severity evaluation (28). In our other MRI study (29), 67.9% of a total of 196 AP patients had mesenterical involvement (MI). Our results demonstrated that the increase in the MI score was associated with an increase in the MRSI score (r=0.722, P<0.001). Therefore, we proposed that a finding of mesenterical effusion could also serve as a supplementary indicator for AP severity assessment (29). On axial and coronary T2-weighted with or without fat-suppressed images, transverse-mesocolon and mesenterical involvement (edema, thickening, and effusion) are shown as granular or patchy hyperintensity inflammation extension admixed with hypointensity steatonecrosis (Figure 3) involving spaces and inter-planes from the retroperitoneal spaces (anterior pararenal spaces) to the root of mesenteries and subperitoneal spaces of the mesenteries and transverse-mesocolon (28,29). Importantly, if coronal slices are sufficiently wide to study the mesenteries, these changes may also involve the pelvis.
Pancreatic and/or peripancreatic hemorrhage
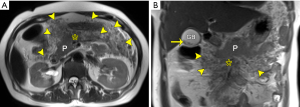
For acute necrotizing pancreatitis, the presence of hemorrhage in or around the pancreas is generally concomitant with parenchymal necrosis on histopathology. In prior reports, hemorrhage was shown in 2–5% of AP patients and was more common in the form of severe necrotizing pancreatitis (13,15). MRI is superior to CT in denoting hemorrhagic AP. This is because hemorrhage-related MRI signal changes may be sustained for a long time, along with different MRI characteristics of hemorrhage at various periods (13-15). Previous studies reported that hemorrhagic fat necrosis with abnormal hyperintense at T1-weighted fat-suppressed imaging was correlated to extremely poor prognosis (30,31). In agreement with their results, we recently conducted a study on MRI features of hemorrhage in AP (pancreatic hemorrhage and hemorrhagic fat necrosis) and correlated the extent of hemorrhage with MRSI and APACHE II scores (14). Our results indicated that hemorrhage in AP (pancreatic hemorrhage and hemorrhagic fat necrosis) was common, with a prevalence of 11.5% among all 539 study patients with AP (14). We also found that the hemorrhage severity index (HSI) in patients with hemorrhage was significantly associated with MRSI (r=0.36, P<0.001) and a higher organ dysfunction rate and longer hospitalization stay compared to those without hemorrhage (14). Thus, the clinical significance of the presence of hemorrhage may correlate with the poor prognosis of AP. Further studies should be conducted to understand the distinction, for one, between acute hemorrhage related to arterial leakage at the early stage of AP, and, for another, hand clot in walled-off necrosis at the late stage of AP without the necessity of urgent treatment.
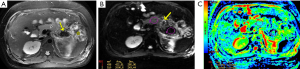
On MR imaging, pancreatic and/or peripancreatic hemorrhage may be admixed with the necrosis of pancreatic and/or peripancreatic tissues, which is visualized as spotted, patchy, or large diffuse regions with hyperintensity on T1-weighted fat-suppressed images (Figure 4). In addition, the DWI and ADC maps may aid in detecting and differentiating between hemorrhage secondary to necrosis and necrosis proper (Figure 5).
Infection complication
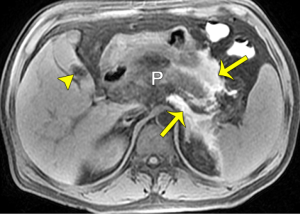
Among AP patients, secondary bacterial infection may appear in the setting of: (I) PN, (II) EPN or PPN (retroperitoneal, or mesenterical fat necrosis), (III) combined necrosis, and (IV) peripancreatic fluid collections (4,13). Generally, infectious necrosis occurs in the course of delayed periods (frequently 2–5 weeks) after the onset of AP (especially necrotizing patterns) (4-8,16). In prior clinical studies, “pancreatic cellulitis” and “pancreatic abscess” had been described as typically infectious complications in patients with AP (13,15). However, these two terms have been abolished based upon the 2012 revised Atlanta Classification (4). Accordingly, four new terms—the acute peripancreatic fluid collection with infection, pseudocyst with infection, the acute necrotic collection with infection, and walled-off necrosis with infection—can be utilized for AP patients with secondary infection conditions (4). Once infectious complications emerge, they induce a high mortality risk for AP patients with a mortality rate of 32% (5-8).
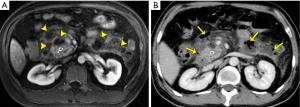
MRI can reflect the range of the involvement of pancreatitis-related infection. The associated findings indicating local infection include ring-like and/or separated enhancement, non-enhancing areas within the major central part of the lesion, and most importantly, the sign of bubble or liquid-gas level within a lesion (13,15). However, gas within a collection is visible in only 12–18% of patients with AP (7,21,22). In our experience, about 50% of AP cases with infection lack typical bubble-sign regardless of ultrasound, CT, or MRI imaging, and small quantities of bubble or gas in a lesion may not be easily identified even on MRI (Figure 6). Consequently, we emphasize that infection complications associated with AP should be suspected when individuals suffer from AP and manifest symptoms related to fever, tachycardia, abdominal distention, systemic infection poisoning reaction, and the long-term presence of non-encapsulated or encapsulated collections in MRI (Figure 6). DWI can additionally provide the information on differentiation infected collections from sterile collections, which may present with more diffusion restriction in central portions of infected collections over their sterile counterparts (16,17). However, measured ADC values could overlap for subjects with or without infection (16,17). On the other hand, invasive intervention for AP-related collections generally results in post-procedural gas, a common imaging feature not as indicative of infection, which should be interpreted by radiologists as a sign to proceed with caution (32,33). Fine-needle aspiration biopsy of a lesion may be needed in some cases for the confirmed diagnosis and subsequent treatment (32-34).
Local complications on pancreatic fluid collections
Acute peripancreatic fluid collection versus acute necrotic collection
Imaging, especially the CT and MRI modalities, plays an important role in assessing extrapancreatic changes such as local complications that do not involve the pancreas itself. Using new terms from the 2012 revised Atlanta Classification (4), acute peripancreatic fluid collection (APFC) or acute necrotic collection (ANC) is recommended for use within 4 weeks of AP onset in edematous pancreatitis or necrotizing pancreatitis, respectively. Although CT has evolved as a first-option tool to identify local complications in AP non-invasively, it is difficult to discriminate between APFC and ANC in the early phase depending on CT alone because of the limited sensitivity of CT in showing the necrosis debris of peripancreatic tissue (4-9). Compared with CT, MRI has an excellent resolution for soft tissue and can help evaluate necrosis solid components localized in these collections accurately; it thus has a valuable role for the differential diagnosis of APFC and ANC in the early phase of AP.
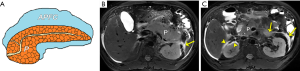
On MRI, APFC exhibits a homogeneously non-encapsulated fluid feature without extension into the pancreatic parenchyma, along with an absence of solid necrosis components (Figure 7). If a pancreatic non-encapsulated collection spread within the pancreatic parenchyma is identified within the first 4 weeks after an episode of necrotizing pancreatitis, the corresponding terminology for ANC should be used (4-8). On MR images, ANCs, surrounding the pancreas, within the pancreas, or both, are observed as heterogeneous and non-encapsulated fluid collections frequently situated in the retroperitoneal spaces, containing variable hypointense necrosis debris (Figure 8). Furthermore, MRCP can aid in detecting pancreatic ductal disruption (frequently reflecting necrosis of the main pancreatic duct) and communication with an intraparenchymal ANC cavity (7,18,19). APFC and ANC usually resolve spontaneously and may not routinely warrant drainage (34).
Pseudocyst versus walled-off necrosis
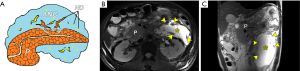
As APFC or ANC continues after treatment, both may gradually decrease in size and resolve or be reabsorbed completely (5-7,34). However, APFC or ANC in some patients may progress into well-circumscribed encapsulation collections beyond 4 weeks after the onset of AP, which can be considered pseudocyst or walled-off necrosis (WON) (4). According to the standards of the 2012 revised Atlanta Classification, AP-associated pancreatic pseudocyst is a very uncommon type of local complication. This is because it is strictly defined as a peripancreatic (not intrapancreatic) well-defined and encapsulated cystic lesion on CECT in patients who suffer from edematous pancreatitis only instead of post-necrotic pancreatitis (4-8). The pancreatic pseudocyst contains homogeneous fluid alone filled with amylase-rich juices (devoid of necrotic solid components) and is surrounded by a thin rim of fibrous pseudo-capsule on CECT (5-8). MRI features of a typically peripancreatic pseudocyst comprise a round-to-oval fluid exudation with the mainly liquid signal of usually T1 hypointensity and T2 hyperintensity (Figure 9), and smooth and symmetric walls with a barely perceptible or uniformly thick finding, and target-like enhancement of walls if enhanced (13,35,36). The identification of dependent necrotic debris presenting as hypointense material with variable shape or size among hyperintense fluid on T2-weighted fat-suppressed images can more confidently be used to exclude pseudocyst while indicating the diagnosis of WON (discussed below). In fact, the value of perceived internal debris can indicate the diagnosis of pseudocysts (35), but all of these lesions, under the 2012 revised Atlanta Classification, would be renamed as “WON”.
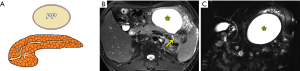
On the other hand, both extrapancreatic and intrapancreatic organized fluid cavities occurring in post-necrotic pancreatitis after 4 weeks of AP onset are designated with the term “WON” (4-7). A WON is considered the necrosis of fat, being mainly solid. The clinical significance for making a distinction between WON and a pancreatic pseudocyst is related to the management strategy for the AP patients. Usually, patients with pancreatic pseudocysts have been treated conservatively or with percutaneously external catheter drainage alone under CT or ultrasound guidance (6,7,34), whereas individuals with WON may undergo more aggressive intervention, such as endoscopic debridement or even surgical necrosectomy depending on the amount of necrotic debris (35-38).
In fact, a prior study reported that the solid material could be detected by using CT in only 45% of patients with WON (7); whereas, the solid material could be identified via MRI in almost all of the WON cases through our clinical practice, especially for a small amount of solid material. Specific MRI findings of WON often include round-to-oval encapsulated and heterogeneous collections with discrete and irregular thick walls (≥3 mm) dominated by T2 hypointensity and hypointense-dependent and wall-attached components (pancreatic and fat tissue fragments/necrotic debris) on T2-weighted fat-suppressed MR images (Figure 10). The WON’s walls generally present as distinct target-like enhancement in CE-MRI (Figure 10). In addition, WON also more commonly involves the parenchyma of the pancreas on imaging and causes the marked deformation and discontinuation of the pancreas (5-8).
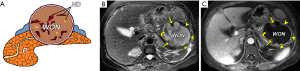
Patients with WON may be asymptomatic (about 50%) or manifest with a variety of clinical manifestations (4-7). Furthermore, sequential WON-associated complications may emerge in some cases, such as gastric obstruction, bleeding due to adjacent vessel erosion, intestinal fistula, secondary infection, and disconnected pancreatic duct syndrome (13,16,18,33,39).
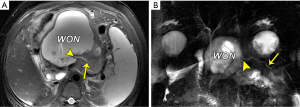
The term “disconnected pancreatic duct syndrome” was coined to describe an anatomic location where there is a lack of pancreatic duct continuity between an isolated viable pancreatic segment and the gastrointestinal tract (18,19). Persistent fistula and inflammatory WONs subsequently arise and are unlikely to resolve with conservative drainage management. The MRI hallmarks of a disconnected pancreatic duct include a large intrapancreatic WON of a section of pancreatic head, neck, or body, as well as a viable portion of the distal body or tail (Figure 11). When visible, the pancreatic duct enters the WON at an angle of approximately 90° (Figure 11) (18,19). Finally, the accompanying management issues with this syndrome may involve the removal of viable disconnected pancreatic tissues (distal pancreatectomy with or without splenectomy) or the preferred surgical therapy of a median pancreaticojejunostomy to drain the segment.
Summary
Multimodal MR images that integrate a series of sequences, including T1- and T2-weighted imaging, DWI with ADC map, MRCP, and DCE-MR imaging, have been increasingly used for evaluating AP before therapeutic plan-making. When trying to diagnose AP using multiparametric MRI, we suggest that the following considerations should be taken into account.
(I) For imaging of interstitial edematous pancreatitis, MRI has a better manifestation than CT to identify the morphology and signal changes in the majority of patients, such as pancreatic edema and interlobular septal abnormalities (11,13). In particular, T2-weighted fat-suppressed images can accurately demonstrate subtle interlobular septal threadlike hyperintensity in the early phase of edematous AP (13,15). (II) For imaging of necrotizing pancreatitis, the combination of T1- and T2-weighted appearances associated with CE-MRI findings can comprehensively assess the necrotic extent and full range of inflammatory spread (4-8). We emphasize that the visualization of non-enhanced pancreatic tissues during the entire CE procedure firmly supports the correct diagnosis for real pancreatic necrosis and not transient pancreatic ischemia. Additionally, MRI may be recommended in the course of more than 3 days (optimum time: 5–7 days) after the onset of AP with regards to the complete maturation of pancreatic necrosis (5-7). However, recent researches have shown that early imaging tests (less than 3 days of AP onset) also can provide valuable information on pancreatic necrosis and outcome evaluation (12-15,24-26,28,29). Moreover, a MRSI score is recommended to be calculated as a favorable predictor for the AP severity evaluation in the early phase of the disease (24,25). (III) For the imaging of retroperitoneal and mesenterical changes, the combination of axial and coronal T2-weighted imaging with MRCP offers a comprehensive evaluation of the range of retroperitoneal fat involvement in AP (28,29), which has an advantage over CT scans. (IV) For differentiation among major local complications, it is easier for MRI to identify the characteristic heterogeneity of variable solid or non-liquefied material with T2-hypointense in hyperintense fluid collections, as an indication that this is ANC or WON rather than APFC or pseudocyst (6-8,40).
Although contrast-enhanced CT is currently the first-line imaging method to assess patients with AP, recent advances favor multiparametric MRI for the comprehensive evaluation of AP pathology. The recognition of the appropriate imaging timing and utilization of proper terminology for reporting imaging features by both clinicians and radiologists can aid in definitive diagnosis and effective treatment planning for AP. Overall, MRI is an excellent tool for detecting and differentiating common local complications following AP.
Acknowledgments
Funding: Two grants from Health Department of Sichuan Province (No. 110296) and Education Department of Sichuan Province of China (No. 15ZB0191).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Vidarsdottir H, Möller PH, Vidarsdottir H, Thorarinsdottir H, Björnsson ES. Acute pancreatitis: a prospective study on incidence, etiology, and outcome. Eur J Gastroenterol Hepatol 2013;25:1068-75. [Crossref] [PubMed]
- Tenner S, Baillie J, DeWitt J, Vege SS. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol 2013;108:1400-15. [Crossref] [PubMed]
- Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology 2013;144:1252-61. [Crossref] [PubMed]
- Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS. Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013;62:102-11. [Crossref] [PubMed]
- Murphy KP, O'Connor OJ, Maher MM. Updated imaging nomenclature for acute pancreatitis. AJR Am J Roentgenol 2014;203:W464-9. [Crossref] [PubMed]
- Thoeni RF. The revised Atlanta classification of acute pancreatitis: its importance for the radiologist and its effect on treatment. Radiology 2012;262:751-64. [Crossref] [PubMed]
- Bollen TL. Acute pancreatitis: international classification and nomenclature. Clin Radiol 2016;71:121-33. [Crossref] [PubMed]
- Meyrignac O, Lagarde S, Bournet B, Mokrane FZ, Buscail L, Rousseau H, Otal P. Acute Pancreatitis: Extrapancreatic Necrosis Volume as EarlyPredictor of Severity. Radiology 2015;276:119-28. [Crossref] [PubMed]
- Arvanitakis M, Delhaye M, De Maertelaere V, Bali M, Winant C, Coppens E, Jeanmart J, Zalcman M, Van Gansbeke D, Devière J, Matos C. Computed tomography and magnetic resonance imaging in the assessment of acute pancreatitis. Gastroenterology 2004;126:715-23. [Crossref] [PubMed]
- Arvanitakis M, Koustiani G, Gantzarou A, Grollios G, Tsitouridis I, Haritandi-Kouridou A, Dimitriadis A, Arvanitakis C. Staging of severity and prognosis of acute pancreatitis by computed tomography and magnetic resonance imaging-a comparative study. Dig Liver Dis 2007;39:473-82. [Crossref] [PubMed]
- Kim YK, Ko SW, Kim CS, Hwang SB. Effectiveness of MR imaging for diagnosing the mild forms of acute pancreatitis: comparison with MDCT. J Magn Reson Imaging 2006;24:1342-9. [Crossref] [PubMed]
- Peng R, Zhang XM, Ji YF, Chen TW, Yang L, Huang XH, Chi XX. Pancreatic duct patterns in acute pancreatitis: a MRI study. PLoS One 2013;8:e72792. [Crossref] [PubMed]
- Xiao B, Zhang XM, Tang W, Zeng NL, Zhai ZH. Magnetic resonance imaging for local complications of acute pancreatitis: a pictorial review. World J Gastroenterol 2010;16:2735-42. [Crossref] [PubMed]
- Tang MY, Chen TW, Bollen TL, Wang YX, Xue HD, Jin ZY, Huang XH, Xiao B, Li XH, Ji YF, Zhang XM. MR imaging of hemorrhage associated with acute pancreatitis. Pancreatology 2018;18:363-9. [Crossref] [PubMed]
- Ji YF, Zhang XM, Mitchell DG, Li XH, Chen TW, Li Y, Bao ZG, Tang W, Xiao B, Huang XH, Yang L. Gastrointestinal tract involvement in acute pancreatitis: initial findings and follow-up by magnetic resonance imaging. Quant Imaging Med Surg 2017;7:641-53. [Crossref] [PubMed]
- Islim F, Salik AE, Bayramoglu S, Guven K, Alis H, Turhan AN. Non-invasive detection of infection in acute pancreatic and acute necrotic collections with diffusion-weighted magnetic resonance imaging: preliminary findings. Abdom Imaging 2014;39:472-81. [Crossref] [PubMed]
- Dhaka N, Samanta J, Kochhar S, Kalra N, Appasani S, Manrai M, Kochhar R. Pancreatic fluid collections: What is the ideal imaging technique? World J Gastroenterol 2015;21:13403-10. [Crossref] [PubMed]
- Sandrasegaran K, Tann M, Jennings SG, Maglinte DD, Peter SD, Sherman S, Howard TJ. Disconnection of the pancreatic duct: an important but overlooked complication of severe acute pancreatitis. Radiographics 2007;27:1389-400. [Crossref] [PubMed]
- Gillams AR, Kurzawinski T, Lees WR. Diagnosis of duct disruption and assessment of pancreatic leak with dynamic secretin-stimulated MR cholangiopancreatography. AJR Am J Roentgenol 2006;186:499-506. [Crossref] [PubMed]
- Jain R, Levine M. Correction to the relative risk calculation for gadolinium-enhanced MR imaging and nephrogenic systemic fibrosis. Radiology 2010;255:307-8. [Crossref] [PubMed]
- Balthazar EJ. Acute Pancreatitis: Assessment of Severity with Clinical and CT Evaluation. Radiology 2002;223:603-13. [Crossref] [PubMed]
- Balthazar EJ, Robinson DL, Megibow AJ, Ranson JH. Acute pancreatitis: value of CT in establishing prognosis. Radiology 1990;174:331-6. [Crossref] [PubMed]
- Mortele KJ, Wiesner W, Intriere L, Shankar S, Zou KH, Kalantari BN, Perez A, vanSonnenberg E, Ros PR, Banks PA, Silverman SG. A modified CT severity index for evaluating acute pancreatitis: improved correlation with patient outcome. AJR Am J Roentgenol 2004;183:1261-5. [Crossref] [PubMed]
- Tang W, Zhang XM, Xiao B, Zeng NL, Pan HS, Feng ZS, Xu XX. Magnetic resonance imaging versus Acute Physiology And Chronic Healthy Evaluation II score in predicting the severity of acute pancreatitis. Eur J Radiol 2011;80:637-42. [Crossref] [PubMed]
- Xie CL, Zhang M, Chen Y, Hu R, Tang MY, Chen TW, Xue HD, Jin ZY, Zhang XM. Spleen and splenic vascular involvement in acute pancreatitis: an MRI study. Quant Imaging Med Surg 2018;8:291-300. [Crossref] [PubMed]
- Sun H, Zuo HD, Lin Q, Yang DD, Zhou T, Tang MY, Wáng YXJ, Zhang XM. MR imaging for acute pancreatitis: the current status of clinical applications. Ann Transl Med 2019;7:269. [Crossref] [PubMed]
- Bakker OJ, van Santvoort H, Besselink MG, Boermeester MA, van Eijck C, Dejong K, van Goor H, Hofker S, Ahmed Ali U, Gooszen HG, Bollen TL; Dutch Pancreatitis Study Group. Extrapancreatic necrosis without pancreatic parenchymal necrosis: a separate entity in necrotising pancreatitis? Gut 2013;62:1475e80.
- Chi XX, Zhang XM, Chen TW, Huang XH, Yang L, Tang W, Xiao B. The normal transverse mesocolon and involvement of the mesocolon in acute pancreatitis: an MRI study. PLoS One 2014;9:e93687. [Crossref] [PubMed]
- Chi XX, Zhang XM, Chen TW, Tang W, Xiao B, Ji YF, Huang XH. Magnetic resonance imaging for the normal mesostenium and involvement of the mesostenium in acute pancreatitis. Biomed Res Int 2014;2014:924845.
- Martin DR, Karabulut N, Yang M, McFadden DW. High signal peripancreatic fat on fat-suppressed spoiled gradient echo imaging in acute pancreatitis: preliminary evaluation of the prognostic significance. J Magn Reson Imaging 2003;18:49-58. [Crossref] [PubMed]
- Kim YK, Kim CS, Han YM. Role of fat-suppressed t1-weighted magnetic resonance imaging in predicting severity and prognosis of acute pancreatitis: an intraindividual comparison with multidetector computed tomography. J Comput Assist Tomogr 2009;33:651-6. [Crossref] [PubMed]
- van Baal MC, Bollen TL, Bakker OJ, van Goor H, Boermeester MA, Dejong CH, Gooszen HG, van der Harst E, van Eijck CH, van Santvoort HC, Besselink MG. Dutch Pancreatitis Study Group. The role of routine fine-needle aspiration in the diagnosis of infected necrotising pancreatitis. Surgery 2014;155:442-8. [Crossref] [PubMed]
- Fritz S, Hackert T, Hartwig W, Rossmanith F, Strobel O, Schneider L, Will-Schweiger K, Kommerell M, Büchler MW, Werner J. Bacterial translocation and infected pancreatic necrosis in acute necrotising pancreatitis derives from small bowel rather than from colon. Am J Surg 2010;200:111-7. [Crossref] [PubMed]
- Sarathi Patra P, Das K, Bhattacharyya A, Ray S, Hembram J, Sanyal S, Dhali GK. Natural resolution or intervention for fluid collections in acute severe pancreatitis. Br J Surg 2014;101:1721-8. [Crossref] [PubMed]
- Macari M, Finn ME, Bennett GL, Cho KC, Newman E, Hajdu CH, Babb JS. Differentiating pancreatic cystic neoplasms from pancreatic pseudocysts at MR imaging: value of perceived internal debris. Radiology 2009;251:77-84. [Crossref] [PubMed]
- Miller FH, Keppke AL, Dalal K, Ly JN, Kamler VA, Sica GT. MRI of Pancreatitis and Its Complications: Part 1, Acute Pancreatitis. AJR Am J Roentgenol 2004;183:1637-44. [Crossref] [PubMed]
- Hollemans RA, Bakker OJ, Boermeester MA, Bollen TL, Bosscha K, Bruno MJ, Buskens E, Dejong CH, van Duijvendijk P, van Eijck CH, Fockens P, van Goor H, van Grevenstein WM, van der Harst E, Heisterkamp J, Hesselink EJ, Hofker S, Houdijk AP, Karsten T, Kruyt PM, van Laarhoven CJ, Laméris JS, van Leeuwen MS, Manusama ER, Molenaar IQ, Nieuwenhuijs VB, van Ramshorst B, Roos D, Rosman C, Schaapherder AF, van der Schelling GP, Timmer R, Verdonk RC, de Wit RJ, Gooszen HG, Besselink MG, van Santvoort HC. Dutch Pancreatitis Study Group. Superiority of Step-up Approach vs Open Necrosectomy in Long-term Follow-up of Patients With Necrotizing Pancreatitis. Gastroenterology 2019;156:1016-26. [Crossref] [PubMed]
- van Brunschot S, van Grinsven J, van Santvoort HC, Bakker OJ, Besselink MG, Boermeester MA, Bollen TL, Bosscha K, Bouwense SA, Bruno MJ, Cappendijk VC, Consten EC, Dejong CH, van Eijck CH, Erkelens WG, van Goor H, van Grevenstein WMU, Haveman JW, Hofker SH, Jansen JM, Laméris JS, van Lienden KP, Meijssen MA, Mulder CJ, Nieuwenhuijs VB, Poley JW, Quispel R, de Ridder RJ, Römkens TE, Scheepers JJ, Schepers NJ, Schwartz MP, Seerden T, Spanier BWM, Straathof JWA, Strijker M, Timmer R, Venneman NG, Vleggaar FP, Voermans RP, Witteman BJ, Gooszen HG, Dijkgraaf MG, Fockens P. Dutch Pancreatitis Study Group. Endoscopic or surgical step-up approach for infected necrotising pancreatitis: a multicentre randomised trial. Lancet 2018;391:51-8. [Crossref] [PubMed]
- Fischer TD, Gutman DS, Hughes SJ, Trevino JG, Behrns KE. Disconnected pancreatic duct syndrome: disease classification and management strategies. J Am Coll Surg 2014;219:704-12. [Crossref] [PubMed]
- Rana SS, Sharma RK, Gupta P, Gupta R. Natural course of asymptomatic walled off pancreatic necrosis. Dig Liver Dis 2019;51:730-4. [Crossref] [PubMed]


