
Percutaneous cryoablation for the treatment of liver cancer at special sites: an assessment of efficacy and safety
Introduction
Liver cancer is one of the most common malignant tumors in China with high morbidity and mortality rates. Although surgical resection is the best treatment, most patients are in advanced stage or have no indication of surgery until they are in hospital. While minimally invasive treatment would be as effective as surgical resection in those patients with a diameter of a single tumor less than 5 cm; a number of lesions less than 3 with the maximum diameter of single lesion less than 3 cm; without the invasion of blood vessels, bile ducts, adjacent organs, and distant metastasis). The main treatment modalities of minimally invasive operation on unresectable liver cancer are as follows: radiofrequency ablation (RFA), microwave ablation (MWA), cryoablation (CA), laser ablation (LA), irreversible electroporation (IRE), and percutaneous ethanol injection (PEI). RFA is most widely used and plays an important role in the treatment of unresectable liver cancer. However, RFA has some disadvantages, such as incomplete ablation for perivascular tumor (1), the risk of thrombosis, and poor surgical comfort. Like RFA, MWA also belongs to a type of thermal ablation and has the above deficiencies. IRE is a new technique that has been applied in recent years. Although IRE has some advantages such as high tissue ablation selectivity, no heat conduction effect, the sharp edge of the ablation zone, and no damage to the vessel of the adjacent treatment area; it can cause serious complications, including arrhythmia and strong muscle contraction (2-4). PEI is also apt to cause complications, including intra-lesional bleeding, local infections, and pleural effusion. Therefore, the above treatments can benefit some patients, but they are not suitable for the treatment of special site liver cancer (defined as tumor adjacent to large blood vessels, extrahepatic organs, and important structures). As a new modality of treatment, CA has certain advantages such as visible ice ball, good curative effect, activation of anti-tumor immunity, and low incidence of complications (5,6), especially in the treatment for the patients of liver cancer at a special site. Up to now, there has been little research that has focused on the treatment by CA for special site liver cancer. Therefore, the purpose of our study was to investigate the safety, feasibility, and effectiveness of CA in the treatment for special site liver cancer.
Methods
Patients
This retrospective study was approved by the Ethics Committee of Affiliated Changhai Hospital of Second Military Medical University at a single center. Informed consent was waived. Sixty-six patients underwent CA for the treatment of liver cancer between July 2015 and April 2018 at the Affiliated Changhai Hospital. All the patients were hospitalized. Among them, 51 patients were diagnosed as liver metastases from the colon or rectum with histological confirmation, and 15 patients were diagnosed as hepatocellular carcinoma (HCC) (7). The inclusion criteria of clinical data were as follows: (I) liver function at Child-Pugh class A or B; (II) the primary tumor controlled in the patient with metastatic tumor; (III) without portal vein tumor thrombus and extrahepatic metastasis; (IV) suitable path to percutaneous puncture; (V) no serious organic diseases of heart, kidney and brain; (VI) normal range of prothrombin time and platelet count. The exclusion criteria were as follows: (I) patient refused the treatment of CA; (II) distant metastasis before surgery; (III) large amount of ascites.
Definition of special site liver cancer
Special site liver cancer was defined as the tumor directly abutting surrounding structures (such as the liver capsule, gallbladder, vessel, diaphragm, intestine, and adrenal gland) with a maximum distance of 1.0 cm. The distance (from the edge of tumor to surrounding structures) was analyzed on axial and coronal reformat images at the imaging post-processing station. Contrast-enhanced magnetic resonance (MR) imaging was performed before the procedure in all patients. The characteristics of the tumor, liver capsule, and adjacent organs were reviewed on MR imaging by three abdominal radiologists, respectively. All the decisions of imaging features were made in consensus.
Inclusion criteria were as follows: (I) fewer than 3 hepatic tumors; (II) a small solid liver tumor (the maximum diameter ≤3.5 cm); (III) the distance between tumor and liver capsule (≤0.5 cm); (IV) the distance between the tumor and surrounding organs (≤1.0 cm); (V) perivascular tumor (the tumor was in contact with portal veins or hepatic vein which the diameter was no less than 0.3 cm in axial imaging) was defined according to the previous literature (8-13); (VI) if the tumor was adjacent to 2 organs or vessels at the same time, with the nearest organ or vessel being used as a reference.
CA technique
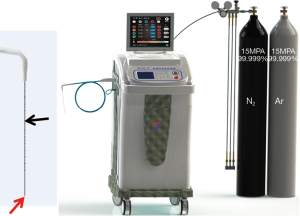
According to the enhanced MR images, the puncture route and CA plan were made before the procedure. All the CA procedures were performed in a standard computed tomography (CT) room. The guided device of imaging was multi-slice spiral CT (SOMATOM, 16 slice spiral CT), while the real-time ultrasound was used when the tumors were not displayed on CT. The operator was an interventional radiologist who had experience for the treatment of liver cancer for more than 5 years. All patients received subcutaneous anesthesia with 5% lidocaine. The CA device (AT-2008-II, AccuTarget MediPharma, Shanghai, China) (Figure 1) had a working pressure of 8,268–10,335 kPa (1,200–1,500 psi) with argon and Nitrogen as the refrigerant. The length of cryoprobes and the diameter of the cutter head were 17.5 and 0.15 cm, respectively. The length of the targeted area was 3.0 cm. According to imaging findings on CT or US during operation, the injection site, puncture route, patient’s position (supine or prone), injection angle, and the amount of freezing were established. The time of CA procedures and the number of cryoprobes were determined according to the size, number, and geometric shape of the tumor. In accordance with the equipment manufacturer’s recommendations, the temperature of each procedure was between −140 and −160 °C with a duration of 13–15 min, and the thawing time was about 3 min with the highest temperature of 50 °C.
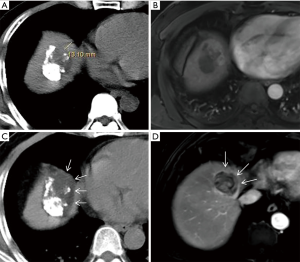
The boundary of ice ball (multi-plane reconstruction was used to measure the distance between the edge of the ice ball and the outermost boundary of the tumor) at least exceeded 0.5 cm beyond the periphery of the tumor. When the distance of the area between the tumor and the hepatic capsule was less than 0.5 cm (Figure 2), the measured distance was calculated based on the distance from the non-hepatic capsule side of the tumor to cryoprobe. The procedure was finished when the ice-ball completely covered the entire tumor on the ultrasound (US) or CT (the tumor was completely enclosed by the peripheral low-density area of ice-ball including peripheral partial normal hepatic tissue surrounding the tumor on the images, although in some cases it was too difficult to obtain a satisfactory ablative margin). Blood pressure, oxygen saturation, heart rate, and electrocardiographic monitoring were performed during operation. Hemostasis, liver function protection, and anti-infection treatment were actively performed after operation. At the same time, urinary myoglobin level was also monitored in case of renal failure caused by the increased level of myoglobin after operation (11).
Follow-up and evaluation
The main method of follow-up was to observe the progress of the tumor by dynamic enhancement MR (Figure 3). The first follow-up period was 1 month after the operation and every 3 months thereafter. If local recurrence or extrahepatic metastasis occurred during the follow-up period, the next treatment was formulated based on the tumor and other conditions of the patient.
Technical success was defined as the tumor being completely included in the hypodense ablation zone in comparison with the parenchyma of the normal liver tissue. Following the procedure on CT imaging, multiplanar reconstruction technique was simultaneously used to observe the tumor. The technical efficacy was defined as no significant enhancement found on dynamic enhancement MR imaging 1 month after the procedure.
Long-term potentiation (LTP) was characterized as the findings of new enhancing lesions in or around the ablation zone on MR imaging. The definition of aggressive intrasegmental recurrence (more than 3 nodules simultaneous or invasive tumor recurrence in the hepatic segment where the tumor was treated) was consistent with previous literature (14).
Intraoperative complications were mainly observed whether there were intrahepatic hemorrhage, pneumothorax, and rupture of peripheral organs. Postoperative complications were classified into intrahepatic, hepatic capsulafibrosa-related, and extrahepatic liver according to the different locations. The grade of severity was evaluated according to whether clinical intervention was needed using the following grading criteria: severe complications referred to whether life was at risk or with severe sequelae, moderate complications referred to whether recovery was required after clinical intervention, and mild complications referred to without clinical intervention). The previous reports (15) were also referenced. Intrahepatic complications mainly included biloma, the injury of the bile duct, and blood vessel. The capsula-fibrosa-related complications were hepatic capsule injury (defined as incomplete and smooth liver capsule on CT or MR enhanced images), subcapsular implantation, and metastasis of the tumor. Extrahepatic complications mainly included the assessment of surrounding organs damaged by CA. If multiple complications occurred during CA simultaneously, the most serious complication was to be considered. Pleural effusions and perihepatic fluid were not considered to be complications, according to the previous reports (16,17).
A scale rated from 0 to 10 was used to assess pain score (18,19). All of the patients were informed of the treatment process and were told how to express the degree of pain during and after the operation (0 = no pain, 1–3 = mild pain, 4–7 = moderate pain, and 8–10 = severe pain).
Statistical analysis
SPSS 22.0 software was used for statistical analysis. Standards deviations and means were used as descriptive statistics for evaluating the baseline characteristics of the study population. The cumulative LTP rate was estimated by the Kaplan-Meier method. The observational period of recurrence analysis was defined as the interval between the first treatment and death or the date of the last follow-up visit before April 2018. A P value of
Results
Patients and tumors
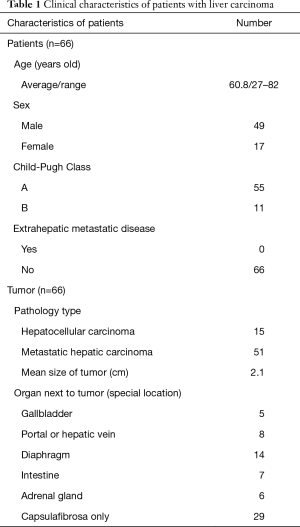
The characteristics of 66 patients and tumors are presented in Table 1. Among the 66 patients, 15 were HCC, and 51 were metastatic carcinoma (primary tumors were from the colon and rectum in 30 and 21, respectively). Sixty-three patients had a single tumor, and 3 patients had 2 tumors. The numbers of patients with Child-Pugh grades at A and B were 55 and 11, respectively. In the 11 patients with Child-Pugh score B, the ascites were not found at the edge of the lesion in 8 patients, and we eliminated the impact of ascites by changing the position during the treatment in the remaining 3 patients. None of the patients had extrahepatic metastasis. The range of tumors size was from 0.8 to 3.5 cm in diameter (M ± SD, 2.1±0.80). The number of tumors adjacent to the hepatic capsule and adjacent to the gallbladder, portal or hepatic vein, diaphragm, intestine, and the adrenal gland was 29, 5, 8, 14, 7, and 6, respectively. Depending on the size and location of the tumors, 1 to 3 (mean, 1.2) cryoprobes were used.
Full table
Technical success and technique effectiveness
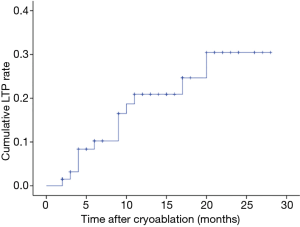
In our study, the technical success rate was 100%. The primary technique efficacy rate was 65 in 66 patients (98.5%) at a one-month follow-up by dynamic enhancement MR examination, and the peripheral organ injury on MR imaging was not found. The median follow-up time was 14 months (range, 2–28 months). During the follow-up, local tumor recurrence and subcapsular implantation metastases were found in 13 patients. Among them, 1 patient was treated with transarterial chemoembolisation (TACE) and iodine-125 seed implantation because of local tumor progression accompanied by intrahepatic metastasis and portal vein tumor thrombus. Two patients underwent CA again, and the remaining patients were treated with RFA, TACE, and targeted drug therapy. The cumulative LTP rates were 10.2% [95% confidence interval (CI): 2.4–18.0%], 16.5% (95% CI: 6.5–26.5%), 20.9% (95% CI: 9.7–32.0%) and 30.5% (95% CI: 14.0–46.6%) at 6, 9, 15, and 24 months, respectively (Figure 4).
Complications
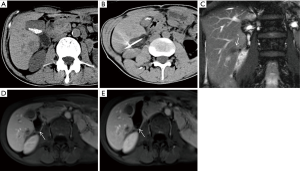
The classification of complications is presented in Table 2. No patients died, and no serious complications occurred during the procedure. Subcapsular hemorrhage and pneumothorax were found in 2 and 1 patients, respectively. They resolved spontaneously on the third day after the procedure. Intrahepatic complications, including abscess, intrahepatic implantation metastasis, and hepatic infarction, were not observed in our study. Biloma (0.5 cm in diameter) was found in 1 patient during the first month after the procedure, and there was no evidence of progressive enlargement during the follow-up period. Hepatic capsular injury (Figure 5) and subcapsular implantation metastasis were found in 13 and 1 patients, respectively. No clinical symptoms associated with capsular injury were found in the first year during the follow-up. CA was performed again at 6 months in the patient with subcapsular implantation metastasis and no recurrence occurred. Slight pneumothorax was found in 2 patients, chest tightness, and chest pain was not observed during the follow-up period after discharge. Abnormities of the intestine, gallbladder, adrenal gland, and other organs were not found on MR imaging at 3 and 6 months, and there were no related clinical symptoms.
Full table
Intraoperative and postoperative visual analog scale (VAS) score
The average procedure duration was 50 min, and all the patients were able to tolerate the pain caused by CA. The VAS score was 2.15±0.63 and 3.25±0.58 during and after operation, and no analgesia was used.
Discussion
At present, RFA is the most widely used technique of minimally invasive treatment in the treatment of unresectable liver tumors (5-7). The mechanism of RFA is that the radiofrequency needle generates and conducts heat to kill the tumor cells. Its safety and efficacy have been described in the previous literature (6,7,20), and the focus of previous studies has mainly been on the treatment of deep liver tumors. For special site liver cancer, RFA often failed to achieve complete ablation coverage of the tumor resulting in tumor residue or recurrence; meanwhile, they would increase the risk of complications of the adjacent organs or large vessels (21,22). Instead, the mechanism of CA is to form ice ball by lowering local tissue temperature, which avoids the adverse effect of a thermal energy source and improves patient comfort during the procedure; indeed, CT or MR imaging show that the margin of ice ball is clearer than in RFA. In addition, CA leads to fewer complications.
At present, there are few studies of CA on the treatment of liver cancer located in special locations, and the data on the success rate and effectiveness are limited. The technical success rate was 100% in our study. According to previous reports (23-28), the technical success rate of hepatic tumors adjacent to the vessels, gallbladder, intestine, and diaphragm is 96.6%, 90%, 93%, and 79% with the use of RFA or CA. Due to the careful analysis of MR images before surgery and the use of multi-plane reconstruction techniques during the procedures, our technical success rate was higher than those previously reported. The primary technique efficacy rate of our study was 98.5% at a one-month follow-up and was similar to previous reports.
In our study, the median LTP was 9 months (range, 3–24 months), the cumulative LTP rates at 6, 9, 15, and 24 months were 10.2%, 16.5%, 20.9%, and 30.5%, respectively. The result of our study was slightly worse than those in previous reports (23,24,29-31) owing to the complexity and particularity of its location. CA was feasible and effective in the treatment of special site liver cancer.
For complications, the incidences of minor and severe complications in RFA were 2.2–5.7% and 1.7–6.3% (32,33), respectively. Compared with RFA, no serious complications and additional severe complications were observed in our study, which shows that CA is safe in the treatment of special site liver cancer.
The damage of small vessels and the bile duct are the main factors of post-operative intrahepatic complications, including segmental infarction of liver, biloma, and arteriovenous fistula. RFA and MWA etc. damage tumor cells by releasing heat, and they also damage the vascular endothelium resulting in mural thrombosis or vascular stenosis (34). The formation of a mural thrombus or vascular stenosis is an important cause of hepatic infarction, and severe cases may lead to acute hepatic failure. In addition, local hepatic hypoperfusion is also an important factor in prompting tumor recurrence. In our study, mural thrombus and vascular stenosis were not found. The injury of the bile duct wall is mainly caused by mechanical injury or hypothermia, which is an important factor in the formation of biliary fistula or biloma. Acute biliary fistula can cause severe abdominal pain and peritonitis, and surgical treatment is required in severe conditions. Biloma occurred in 1 patient with no progressive increase during the follow-up in our study, and no further treatment was required.
Furthermore, the incidence of disseminated intravascular coagulopathy (DIC) and myoglobinemia are associated with tumor size, meaning that larger tumors have a higher incidence (35). The occurrence of myoglobinemia is also related to preoperative renal function and the damage degree of the adjacent organ wall. To prevent serious complications, some researchers used lower ablation time combined RFA with TACE (36), which could increase the risk of liver infarction. Therefore, we suggest that the size of the tumor is critical in the selection of minimally invasive treatment for liver cancer. DIC and myoglobinemia did not occur in our study. We thus believe that CA is safe and reliable in the treatment of liver cancer with a diameter of less than 3.5 cm.
The major hepatic-capsule-related complications were liver capsule injury and subcapsular implantation metastasis. The liver capsule consists of a compact fibrous structure with strong plasticity which makes it difficult to be damaged. When the tumor invades the liver capsule, or it is pulled by mechanical reasons resulting in the reduction of toughness, it may be damaged or ruptured more easily. In our study, the proportion of patients with liver capsule injury was 19.6%. During the follow-up, the liver capsule manifested as discontinuous enhancement and pitted sign on MR images without any related clinical symptoms. In addition, the patients with liver capsule involvement are more prone to have subcapsular implantation metastasis, and it occurred in one of our patients. The possible explanation is that the tumor cells spread along the liver capsule by subcapsular effusion or hemorrhage during the procedure, but this supposition needs to be confirmed by further research.
Extrahepatic complications are mainly caused by the injury of surrounding organs, and the diaphragm is most often involved. In the treatment of subdiaphragmatic tumors with RFA, the thermal damage rate of the diaphragm was up to 55.2%, and the rate of gastrointestinal perforation was 0.3% to 1.0%. The colonic wall was more easily damaged and perforated owing to a relatively thin and fixed position (37-39). Kang et al. (28) reported a higher damage rate of the diaphragm and a lung injury incidence of 25% with the use of RFA. Choi et al. (27) reported one patient with a small perihepatic abscess with the use of RFA. Compared with RFA, CA has less damage to extrahepatic tissues or structures. Fairchild et al. (24) reported that CA was safe for the treatment of hepatic tumors adjacent to the gallbladder even when the ice ball extended as far as 1.8 cm into the gallbladder lumen. Li et al. (40) reported that abdominal hemorrhage, hepatic abscess, biliary fistula, gastrointestinal perforation, and myoglobinuria were not found in the treatment of hepatic tumors next to the gastrointestinal tract with CA. Also, in our study, significant injury of the extrahepatic organ did not occur, and no serious complications were found. Therefore, we believe that the damage to extrahepatic organs by CA is significantly less than that done by RFA, and CA is more suitable for the treatment of liver cancer in a special location. In addition, CA for the treatment of special site liver cancer under local anesthesia has certain advantages, such as shorter operation time, fewer anesthesia-related complications, low cost, and rapid recovery.
There are some limitations to our research. First of all, our study was retrospective in nature and used a relatively small cohort. Secondly, the LTP rates and long-term complications could not be accurately assessed owing to the short time of follow-up. Thirdly, some patients had received chemotherapy or TACE before the operation, and the results of the effectiveness were affected to some extent. Fourthly, the CA-induced liver tissue necrosis process could have been better evaluated by functional imaging techniques (41,42), which we did not perform. Finally, the CA device is less commonly used at present; therefore, a greater amount of data is needed to verify its purported advantage.
In conclusion, our research suggests that CA is feasible for the treatment of special site liver cancer and does not increase the incidence of complications. We will continue to explore the long-term efficacy of patient treatment in our study and conduct a larger sample and randomized controlled trial in the future.
Acknowledgments
Funding: National Key Specific Clinical Project (No: 008011001010).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Ethics Committee of Affiliated Changhai Hospital of Second Military Medical University. The requirement for individual consent was waived by the ethics committee because of the retrospective nature of the study.
References
- Wright AS, Sampson LA, Warner TF, Mahvi DM, Lee JFT. Radiofrequency versus microwave ablation in a hepatic porcine model. Radiology 2005;236:132-39. [Crossref] [PubMed]
- Zeng J, Liu G, Li ZH, Yang Y, Fang G, Li RR, Xu KC, Niu L. The Safety and Efficacy of Irreversible Electroporation for Large Hepatocellular Carcinoma. Technol Cancer Res Treat 2017;16:120-24. [Crossref] [PubMed]
- Alnaggar M, Qaid AM, Chen J, Niu L, Xu K. Irreversible electroporation of malignant liver tumors: Effect on laboratory values. Oncol Lett 2018;16:3881-88. [PubMed]
- Niessen C, Thumann S, Beyer L, Pregler B, Kramer J, Lang S. Percutaneous Irreversible Electroporation: Long-term survival analysis of 71 patients with inoperable malignant hepatic tumors. Sci Rep 2017;7:43687. [Crossref] [PubMed]
- Zhang JR. A New Challenge on Clinical Oncology: Argon-Helium Targeted Ablation Therapy. Int J Modern Cancer Ther 2002;25:68-72.
- Rong G, Bai W, Dong Z, Wang C, Lu Y, Zeng Z, Qu J, Lou M, Wang H, Gao X, Chang X, An L, Li H, Chen Y, Hu KQ, Yang Y. Long-term outcomes of percutaneous cryoablation for patients with hepatocellular carcinoma within Milan criteria. PLoS One 2015;10:e0123065. [Crossref] [PubMed]
- Korean Liver Cancer Study Group (KLCSG). 2014 Korean Liver Cancer Study Group-National Cancer Center Korea Practice Guideline for the Management of Hepatocellular Carcinoma. Korean J Radiol 2015;16:465-22. [Crossref] [PubMed]
- Lu DSK, Raman SS, Limanond P, Aziz D, Economou J, Busutti R. Influence of large peritumoral vessels on outcome of radiofrequency ablation of liver tumors. J Vasc Interv Radiol 2003;14:1267-74. [Crossref] [PubMed]
- Kang TW, Lim HK, Lee MW, Kim YS, Choi D, Rhim H. Perivascular versus nonperivascular small HCC treated with percutaneous RF ablation: retrospective comparison of long-term therapeutic outcomes. Radiology 2014;270:888-99. [Crossref] [PubMed]
- Lu DSK, Raman SS, Vodopich DJ, Wang M, Sayre J, Lassman C. Effect of vessel size on creation of hepatic radiofrequency lesions in pigs: assessment of the "heat sink" effect. AJR Am J Roentgenol 2002;178:47-51. [Crossref] [PubMed]
- Glazer DI, Tatli S, Shyn PB, Vangel MG, Tuncali K, Silverma SG. Percutaneous Image-Guided Cryoablation of Hepatic Tumors: Single-Center Experience with Intermediate to Long-Term Outcomes. AJR Am J Roentgenol 2017;209:1381-89. [Crossref] [PubMed]
- Lee SM, Won JY, Lee DY, Lee KH, Lee KS, Paik YH. Percutaneous cryoablation of small hepatocellular carcinomas using a 17-gauge ultrathin probe. Clin Radiol 2011;66:752-59. [Crossref] [PubMed]
- Song KD, Lee MW, Rhim H, Kim Y, Kang TW, Shin SW. Aggressive intrasegmental recurrence of hepatocellular carcinoma after combined transarterial chemoembolization and radiofrequency ablation. AJR Am J Roentgenol 2016;207:1122-27. [Crossref] [PubMed]
- Kang TW, Lim HK, Lee MW, Kim YS, Rhim H, Lee WJ. Aggressive intrasegmental recurrence of hepatocellular carcinoma after radiofrequency ablation: risk factors and clinical significance. Radiology 2015;276:274-85. [Crossref] [PubMed]
- van Lier F, Geest PJVD, Hol JW, Leebeek FW, Hoeks SE. Risk modification for postoperative pulmonary embolism: timing of postoperative prophylaxis. Thromb Res 2012;129:e14-7. [Crossref] [PubMed]
- Mala T. Cryoablation of liver tumours--a review of mechanisms, techniques and clinical outcome. Minim Invasive Ther Allied Technol 2006;15:9-17. [Crossref] [PubMed]
- Poggi G, Riccardi A, Quaretti P, Teragni C, Bernardo G. Complications of percutaneous radiofrequency thermal ablation of primary and secondary lesions of the liver. Anticancer Res 2007;27:2911-6. [PubMed]
- Bodian CA, Freedman G, Hossain S, Eisenkraft JB, Beilin Y. The visual analog scale for pain: clinical significance in postoperative patients. Anesthesiology 2001;95:1356-61. [Crossref] [PubMed]
- Katz J, Melzack R. Measurement of pain. Surg Clin North Am 1999;79:231-52. [Crossref] [PubMed]
- Kim HO, Hwang SI, Hong HP, Yoo CH. Radiofrequency ablation for metachronous hepatic metastases from gastric cancer. Surg Laparosc Endosc Percutan Tech 2009;19:208-12. [Crossref] [PubMed]
- Head HW, Dodd GD, Dalrymple NC, Prasad SR, Hubbard LG. Percutaneous radiofrequency ablation of hepatic tumors against the diaphragm: frequency of diaphragmatic injury. Radiology 2007;243:877-84. [Crossref] [PubMed]
- Llovet JM, Vilana R, Brú C, Bianchi L. ASalmeron JM, Boix L, Ganau S, Sala M, Pagès M, Ayuso C, Solé M, Rodés J, Bruix J. Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatology 2001;33:1124-29. [Crossref] [PubMed]
- Kim R, Kang TW, Cha DI, Song KD, Lee MW, Rhim H. Percutaneous cryoablation for perivascular hepatocellular carcinoma: Therapeutic efficacy and vascular complications. Eur Radiol 2019;29:654-62. [Crossref] [PubMed]
- Fairchild AH, Tatli S, Dunne RM, Shyn PB, Tuncali K, Silverman SG. Percutaneous cryoablation of hepatic tumors adjacent to the gallbladder: assessment of safety and effectiveness. J Vasc Interv Radiol 2014;25:1449-55. [Crossref] [PubMed]
- Chopra S, Dodd GD 3rd, Chanin MP, Chintapalli KN. Radiofrequency ablation of hepatic tumors adjacent to the gallbladder: feasibility and safety. AJR Am J Roentgenol 2003;180:697-701. [Crossref] [PubMed]
- Kim SW, Rhim H, Park M, Kim H, Kim YS, Choi D, Lim HK. Percutaneous radiofrequency ablation of hepatocellular carcinomas adjacent to the gallbladder with internally cooled electrodes: assessment of safety and therapeutic efficacy. Korean J Radiol 2009;10:366-76. [Crossref] [PubMed]
- Choi D, Lim HK, Kim MJ, Kim SH, Lee WJ, Kim SH. Therapeutic efficacy and safety of percutaneous radiofrequency ablation of hepatocellular carcinoma abutting the gastrointestinal tract. AJR Am J Roentgenol 2004;183:1417. [Crossref] [PubMed]
- Kang TW, Rhim H, Lee MW, Kim YS, Choi D, Lee WJ. Radiofrequency Ablation for Hepatocellular Carcinoma Abutting the Diaphragm: Comparison of Effects of Thermal Protection and Therapeutic Efficacy. AJR Am J Roentgenol 2011;196:907-13. [Crossref] [PubMed]
- Chang X, Wang Y, Yu HP, Zhang WH, Yang XL, Guo Z. CT-guided percutaneous cryoablation for palliative therapy of gastric cancer liver metastases. Cryobiology 2018;82:43-8. [Crossref] [PubMed]
- Zhang W, Yu H, Guo Z, Li B, Si T, Yang X. Percutaneous cryoablation of liver metastases from breast cancer: initial experience in 17 patients. Clin Radiol 2014;69:231-8. [Crossref] [PubMed]
- Gao W, Guo Z, Zhang X, Wang Y, Yu H. Percutaneous cryoablation of ovarian cancer metastasis to the liver: initial experience in 13 patients. Int J Gynecol Cancer 2015;25:802-8. [Crossref] [PubMed]
- Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of Focal Liver Tumors with Percutaneous Radio-frequency Ablation: Complications Encountered in a Multicenter Study. Radiology 2003;226:441-51. [Crossref] [PubMed]
- de Baère T, Risse O, Kuoch V, Dromain C, Sengel C, Smayra T. Adverse events during radiofrequency treatment of 582 hepatic tumors. AJR Am J Roentgenol 2003;181:695-700. [Crossref] [PubMed]
- Ng KKC, Lam CM, Poon RTP, Shek TWH, Wong J. Delayed portal vein thrombosis after experimental radiofrequency ablation near the main portal vein. Br J Surg 2004;91:632-9. [Crossref] [PubMed]
- Nair RT, Silverman SG, Tuncali K, Obuchowski NA, Shankar S. Biochemical and hematologic alterations following percutaneous cryoablation of liver tumors: experience in 48 procedures. Radiology 2008;248:303-11. [Crossref] [PubMed]
- Takada Y, Kurata M, Ohkohchi N. Rapid and aggressive recurrence accompanied by portal tumor thrombus after radiofrequency ablation for hepatocellular carcinoma. Int J Clin Oncol 2003;8:332-35. [Crossref] [PubMed]
- Lee EJ, Rhim H, Lim HK, Choi D, Lee WJ, Min KS. Effect of artificial ascites on thermal injury to the diaphragm and stomach in radiofrequency ablation of the liver: experimental study with a porcine model. AJR Am J Roentgenol 2008;190:1659-64. [Crossref] [PubMed]
- Park SY, Tak WY, Jeon SW, Cho CM, Kweon YO, Kim SK. The efficacy of intraperitoneal saline infusion for percutaneous radiofrequency ablation for hepatocellular carcinoma. Eur J Radiol 2010;74:536-40. [Crossref] [PubMed]
- Head HW, Dodd GD, Dalrymple NC, Prasad SR, Hubbard LG. Percutaneous radiofrequency ablation of hepatic tumors against the diaphragm: frequency of diaphragmatic injury. Radiology 2007;243:877-84. [Crossref] [PubMed]
- Li B, Liu C, Xu XX, Li Y, Du Y, Zhang C. Clinical Application of Artificial Ascites in assisting CT-guided Percutaneous Cryoablation of Hepatic Tumors Adjacent to the Gastrointestinal Tract. Sci Rep 2017;7:16689. [Crossref] [PubMed]
- Wang YX, Wang X, Wu P, Wang Y, Chen W, Chen H, Li J. Topics on quantitative liver magnetic resonance imaging. Quant Imaging Med Surg 2019. [Crossref]
- Li YT, Cercueil JP, Yuan J, Chen W, Loffroy R, Wang YX. Liver intravoxel incoherent motion (IVIM) magnetic resonance imaging: a comprehensive review of published data on normal values and applications for fibrosis and tumor evaluation. Quant Imaging Med Surg 2017;7:59-78. [Crossref] [PubMed]


