Changes in cochlear blood flow in mice due to loud sound exposure measured with Doppler optical microangiography and laser Doppler flowmetry
Introduction
Adequate cochlear blood supply is crucial for supplying the high cellular metabolic demand required for proper cochlear function. Cochlear ischemia has been related to several hearing disorders, which include noise-induced hearing loss, age related hearing loss, sudden sensorineural hearing loss, tinnitus and Ménière’s disease (1-9). The study of the cochlear microcirculation can be noteworthy for determining the causes and possible treatments of hearing loss. Understanding the underlying mechanisms of cochlear microcirculation is vital for the management of these pathologies (10).
The ability to study cochlear blood flow (CoBF) in vivo, has been challenging due to the invasiveness of accessing the inner ear and the cochlea, and the limited number of tools available. Histology has provided morphological information that captures a single time-point measurement about the cochlea vasculature; however, its results are difficult to interpret due to its invasiveness and ex vivo analysis (11). Other single time-point methods include the microsphere method, which has been used to study CoBF (12-14); however, it requires a large number of captured spheres for statistical purposes. Corrosion cast provides three-dimensional images of the vasculature; however, it is an invasive ex vivo technique that provides vessel morphology but lacks blood flow information (15). Laser Doppler flowmetry (LDF) is a real-time method that can measure relative changes in blood flow within a volume of tissue (a hemisphere of ~1.5 mm radius), which depends on the source detector separation and the wavelength of light used by the system (16,17). LDF can measure the changes in flow (number of red blood cells in a given volume times the mean velocity); however, its spatial resolution is limited. LDF has been previously and extensively used to measure the changes in CoBF during the occlusion of the stapedial artery and anterior inferior cerebellar artery in rats (18), topical application of vasodilators in rats and guinea pigs (19), loud sound exposure (LSE) in guinea pigs (20,21), and hypoxia in mice (22). Laser speckle contrast imaging, which is a two dimensional (no depth information) real-time method that obtains relative values of the changes in blood flow within a volume of tissue, has been used to study the cochlea due to a systemic hypoxia (23). Doppler optical microangiography (DOMAG) is an imaging modality based on optical coherence tomography (OCT) (24-30). DOMAG is another real-time method which differs from LDF in that it enables the non-invasive measurement of the three dimensional microstructural and microvascular composition of the biological tissues. OCT and DOMAG have previously been used to image the cochlea in vivo (31-33), and used for studies of systemic hypoxia (22,23). However, the study of the relationship between LSE and vascular changes has not yet been investigated with DOMAG.
Currently, there is some ambiguity over the effects of LSE on CoBF. For example, histological studies indicate a decrease in circulation based on the alteration of its vessels, such as endothelial wall swelling, constriction of capillaries and unusual spacing of red blood cells (11,34-36). Using various sound paramaters, studies with LDF have also indicated a decrease in CoBF (20,37). On the other hand, other studies have indicated an increase (12,38-40), or no change (13,14,41) in CoBF.
In this study we used DOMAG and LDF to examine the changes in CoBF in anesthetized mice, subjected to a LSE (119 dB SPL) stimulus which consists of white noise (20 kHz bandwidth) for a one hour period.
Materials and methods
The experiments were performed based on the National Institute of Health guidelines for research animal care and the protocols were approved by the Institutional Animal Care and Use Committee at the University of Washington. Male mice C57BL/6J (Charles River Laboratories, Hollister, CA) approximately 10 to 14 weeks old of age with body weight from 25 to 28 g were used in all experiments.
Surgical exposure of the mouse cochlea
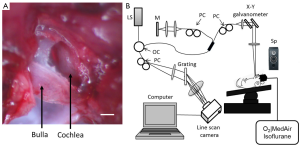
Mice were anesthetized with 1.5% isoflurane in oxygen-enriched air (20% oxygen/80% medical air). The body temperature was maintained between 36.5-37.5 °C by using a feedback rectal probe and a heating pad (Harvard Apparatus, location). The head of the mouse was immobilized onto an imaging platform to minimize movement such that the right cochlea was exposed ventrally through the neck, as previously described (22,23,42). An incision was made down the midline of the neck and the left submandibular gland and posterior belly of the digastric muscle were removed by cauterization. The external carotid artery was ligated inferior to the bifurcation. The positions of the hypoglossal and facial nerves and the sternocleidomastoid muscles were used to identify the location of the boney bulla that contains the cochlea. A small hole was made on the bulla to expose the cochlea, while maintaining the tympanic membrane and vessels intact. As a result only part of the cochlea was properly exposed to image with the DOMAG system, as observed in Figure 1A, while the view of the rest of the cochlea was blocked by the tympanic membrane.
DOMAG
A spectral domain OCT system was used as presented in Figure 1B (43). A broadband superluminescent diode with a central wavelength of 1,310 nm and a spectral bandwidth of 110 nm was used to achieve a theoretical axial resolution of ~7.2 µm in air. The light was split into two beams using a 2×2 optical coupler. The light of one beam was reflected by a mirror (reference arm), and the light from the other beam was reflected by the cochlea (sample arm). In the sample arm, the light was coupled into an optical system, which contained a collimator, a pair of galvo mirrors, and an objective lens (focal length 30 mm), which was used to focus the probing light on the region of interest, and provided a lateral resolution of ~12 µm. The spectrometer contained an InGaAs line scan camera (SUI, Goodrich Corp.) capable of capturing data at a ~92 kHz A-line scan rate. The spectrometer has a spectral resolution of 0.141 nm, providing a measured imaging depth of ~3.0 mm in air. The system dynamic range was measured at ~105 dB at the depth position of 0.5 mm with an incident optical power of 2.5 mW in the sample arm.
The methods used to capture and process the data were based on the DOMAG technique (44). This method enables the extraction of the three-dimensional microvascular images. Briefly, the X-Y galvanometer where used to scan the OCT beam across the sample, with one scanner moving the beam in the X direction, also known as the fast scan or B-scan, and another scanner moving the beam in the Y direction, also known as the slow scan or C-scan. In this study, the camera had an A-line scan rate of 5 kHz. Each B-scan contained 2,000 A-lines that span 1.5 mm. In each experiment we acquired a three-dimensional data set which consisted of 240 discrete locations in the slow scan where 5 B-frames were collected at each location and averaged together. The slow scan had a length of 1.5 mm. The frame rate was set to two frames per second. The data cube of the three-dimensional image was composed of 1,024×2,000×240 (Z-X-Y) voxels, and the total acquisition time was ten minutes (240 locations ×5 frames per location/2 frames per second).
We collected a three dimensional data set at five time points throughout the experiment. LSE was applied for 60 minutes. The first data point was collected before the LSE stimulus, then three data points were collected at 15, 30 and 45 minutes after the initiation of the stimulus, and finally a data point was collected immediately after the noise stimulus was terminated. The time of these data points will be referred to as 0, 15, 30, 45 and 60 minutes, respectively. It is important to note that the collection of a data point took ten minutes. Also, on a separate animal we acquired data with and without the presence of the LSE, and determined that the LSE does not add artifacts to the measurements; therefore, we were confident we could acquire the data while the LSE stimulus was present.
DOMAG is a method that has been used to calculate the axial blood flow velocity inside vessels. DOMAG has previously been validated by using tissue calibration phantoms, and it has been used for several in vivo studies (44). The red blood cell velocity in the axial direction (direction of the incident OCT beam) is derived by the phase difference between adjacent lines. The relationship between the phase difference (Δφ) and the axial velocity (Vz) is given by:
where λ0 is the central wavelength of the light source (1,310 nm), n is the refractive index of the tissue (~1.35) and ΔtA is the time interval between adjacent lines (1/5 kHz =200 µs). The maximum axial velocity measured by our system parameters was ±1.2 mm/s.
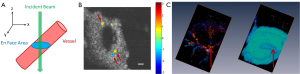
The absolute blood flow rate is an important parameter that can be quantified within blood vessels. An approach to obtain the absolute blood flow velocity consists on obtaining the absolute velocity of blood flow which requires the knowledge of the Doppler angle (θ) (45), where V = abs (Vz/cosθ). The Doppler angle can be calculated by the three-dimensional data set; however it is a cumbersome procedure. A recent approach has been used to determine the absolute blood flow without requiring the knowledge of the Doppler angle (46,47); therefore, simplifying the calculation. The method consists of obtaining a three dimensional map of Vz (x,y,z), from which an en face plane can visualize the vessels, as presented in Figure 2. The absolute blood flow velocity is obtained by integrating the axial flow components over the vessel cross-section on the en face plane by using:
To determine the flow within a vessel, the area of the vessel was manually selected. To minimize the error in the vessel area, each vessel was segmented three times and averaged together, and the value of the area and flow was averaged together.
Figure 2C shows the scalas within the cochlea and the large radiating blood vessels that originate in the apical turn close to the helicotrema. The colors of the vessels indicate the flow direction (red towards the incident OCT beam, and blue away from the incident OCT beam). The radiating vessels resemble the images depicted by Iwagaki et al. who used a resin cast technique (48).
LDF
A LDF system (Periflux PF 2B, Perimed), was used to estimate the changes in flow within a large volume of the cochlea. The tip of the LDF was placed on the cochlea. Given the size of the probe, which evaluates the flow within a hemisphere of ~1.5 mm radius, it was difficult to localize the measurement to only the apical or basal turn. Therefore, we speculate that the measurements cover a volume that contains the whole cochlea (both the apical and basal turns). The sound was turned off during the time when measurements were obtained to avoid the presence of noise artifacts (21). Measurements were obtained every five minutes throughout an hour.
LSE
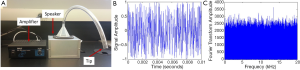
A loud sound speaker system was home built, which produced a broadband white noise (up to 20 kHz), with an intensity of 119 dB SPL. The exposure stimulus was generated by a custom-made white noise source, and delivered through a cone fitted to a plastic tube in the top of the sound exposure box. The tip of the tube was placed within 1 mm of the ear canal. The LSE system and an example of the time and frequency domain of the white noise are presented in Figure 3.
A total of 12 mice were used. There were six mice (three control and three exposed to noise) that were imaged by the DOMAG system. Similarly, a separate cohort of six mice (three control and three exposed to noise) that were measured by the LDF system.
Statistical analysis
Statistical analysis was carried out using Matlab. The differences in flow between the control and noise group using DOMAG were analyzed by a student’s t-test, where it is assumed that the sample populations are normally distributed. The criterion for statistical significance was P<0.05.
Results
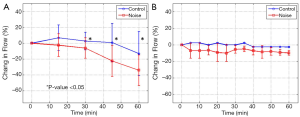
Using the DOMAG system we were able to obtain both structural and microvascular images of the cochlea. For the DOMAG analysis, five of the radiating vessels (as shown in Figure 2B,C) were selected from each cochlea; therefore, there were 15 vessels in the control group and 15 vessels in the noise group. Figure 4A presents the mean and standard deviation of the percentage change in flow of the 15 vessels in each group, referenced to the zero time point.
For the LDF measurements, the data was acquired from three cochleas in each group (control and noise). The mean and standard deviation of the percentage change in flow, referenced to the zero time point, is presented in Figure 4B.
Discussion
This study demonstrates that LSE decreases the blood flow within the cochlea throughout one hour stimulus. Two methods (DOMAG and LDF) were used to validate this hypothesis. It is important to mention that although both methods can determine the changes in CoBF, they probe different tissue volumes. Therefore, the change in blood flow measured by each technique contains a different set of blood vessels. DOMAG can estimate the blood flow within single large vessels in the cochlea; however, LDF can estimate the change in blood flow within a volume of tissue (a hemisphere of ~1.5 mm radius), which contains large vessels and capillaries. Figure 2C shows the three dimensional image of the large radiating vessels that expand from the apical turn. The mouse cochlea has 1.75 turns (49).
DOMAG was used to estimate the blood flow inside individual vessels. An en face analysis was used which allows for the total blood flow estimation without the knowledge of the Doppler angle, which can be challenging to calculate. This method uses Equation 1 and 2. Figure 4A presents the changes in flow for the vessels analyzed using the DOMAG method. In general we note that after 60 minutes there is a blood flow reduction in both groups; however, the noise group has a drastic reduction (~30%) compared to the control counterpart (~10%). The difference between both groups is statistically significant. We speculate that the slight decrease in flow in the control group may be due to the long period of time that the mice were under anesthesia, which could affect part of the blood flow. Another noticeable characteristic is the overlap of the standard deviations observed between the two groups. This indicates that not all vessels within a mouse have the same response to the LSE.
Figure 4B depicts the changes in blood flow averaged throughout the cochlea using LDF. This technique has previously been used in the cochlea of guinea pigs (20), and it has demonstrated that noise contributes to a reduction in CoBF. Our results demonstrate that there is approximately a 10% reduction in blood flow throughout one hour of LSE in the noise group compared to an approximately 0% change in the control group. Given that the size of the mouse cochlea is small, the LDF averages the vessels in both the apical and basal turns. The noise bandwidth was limited to 20 kHz (mostly affecting the apical turn), while the mouse hearing can go up to 75 kHz (50). Therefore, the LSE stimulus only affects a sub-set of the whole cochlea which was averaged together by the LDF, contributing to the smaller reduction in flow compared to the DOMAG method.
Although both DOMAG and LDF indicate a reduction in blood flow due to LSE, the magnitude of change after 60 minutes was different, 30% and 10%, respectively. Also, LDF shows a change within the first five minutes which remains constant through time, while DOMAG presents a strong change at about 30 minutes. The difference in the results may be due to the difference in the vessels analyzed with both techniques.
Conclusions
In this study, we have validated that the cochlea has a reduction in blood flow due to LSE. Both DOMAG and LDF determined that there is a reduction in CoBF of ~30% and ~10%, respectively. The difference between the values determined by these techniques can be explained by the differences in the vessels analyzed by each method. DOMAG analyzes the blood flow in a few selected large vessels in the apical turn, while LDF averages all the vessels and capillaries within a volume of tissue containing the whole cochlea. Further studies should be made to better understand the phenomenon of the change in CoBF due to LSE. Understanding the underlying causes in the reduction of blood flow can aid in improving the diagnosis and treatment for several hearing disorders, such as the ones caused by LSE.
Acknowledgements
This work was supported in part by research grants from the National Institutes of Health (R01DC01201 and R01DC000105). We would like to thank Edward Porsov for the technical help and support received throughout this work. The content is solely the responsibility of the authors and does not necessarily represent the official views of the grant-giving bodies.
Disclosure: The authors declare no conflict of interest.
References
- Perlman HB, Kimura R, Fernandez C. Experiments on temporary obstruction of the internal auditory artery. Laryngoscope 1959;69:591-613. [PubMed]
- Thalmann R, Miyoshi T, Thalmann I. The influence of ischemia upon the energy reserves of inner ear tissues. Laryngoscope 1972;82:2249-72. [PubMed]
- Santi PA, Duvall AJ 3rd. Stria vascularis pathology and recovery following noise exposure. Otolaryngology 1978;86:ORL354-61. [PubMed]
- Ren T, Brown NJ, Zhang M, et al. A reversible ischemia model in gerbil cochlea. Hear Res 1995;92:30-7. [PubMed]
- Lamm K, Arnold W. Noise-induced cochlear hypoxia is intensity dependent, correlates with hearing loss and precedes reduction of cochlear blood flow. Audiol Neurootol 1996;1:148-60. [PubMed]
- Nuttall AL. Sound-Induced cochlear ischemia/hypoxia as a mechanism of hearing loss. Noise Health 1999;2:17-32. [PubMed]
- Mom T, Bonfils P, Gilain L, et al. Origin of cubic difference tones generated by high-intensity stimuli: effect of ischemia and auditory fatigue on the gerbil cochlea. J Acoust Soc Am 2001;110:1477-88. [PubMed]
- Shi X. Physiopathology of the cochlear microcirculation. Hear Res 2011;282:10-24. [PubMed]
- Miller JM, Ren TY, Nuttall AL. Studies of inner ear blood flow in animals and human beings. Otolaryngol Head Neck Surg 1995;112:101-13. [PubMed]
- Mom JCT, Gabrillargues J, Gilain L, et al. Cochlear blood supply: an update on anatomy and function. Fr ORL 2005;88:81-8.
- Hawkins JE Jr. The role of vasoconstriction in noise-induced hearing loss. Ann Otol Rhinol Laryngol 1971;80:903-13. [PubMed]
- Prazma J, Rodgers GK, Pillsbury HC. Cochlear blood flow. Effect of noise. Arch Otolaryngol 1983;109:611-5. [PubMed]
- Hultcrantz E. The effect of noise on cochlear blood flow in the conscious rabbit. Acta Physiol Scand 1979;106:29-37. [PubMed]
- Hultcrantz E, Angelborg C, Beausang-Linder M. Noise and cochlear blood flow. Arch Otorhinolaryngol 1979;224:103-6. [PubMed]
- Tange RA, Wijburg FA. The vasculature of the external wall of the gerbil’s inner ear. Hear Res 1986;23:135-9. [PubMed]
- Miller JM, Marks NJ, Goodwin PC. Laser doppler measurements of cochlear blood flow. Hear Res 1983;11:385-94. [PubMed]
- Goodwin PC, Miller JM, Dengerink HA, et al. The laser Doppler: a non-invasive measure of cochlear blood flow. Acta Otolaryngol 1984;98:403-12. [PubMed]
- Yamamoto H, Tominaga M, Sone M, et al. Contribution of stapedial artery to blood flow in the cochlea and its surrounding bone. Hear Res 2003;186:69-74. [PubMed]
- Tominaga M, Yamamoto H, Sone M, et al. Response of cochlear blood flow to prostaglandin E1 applied topically to the round window. Acta Otolaryngol 2006;126:232-6. [PubMed]
- Thorne PR, Nuttall AL. Laser doppler measurements of cochlear blood flow during loud sound exposure in the Guinea pig. Hear Res 1987;27:1-10. [PubMed]
- Thorne PR, Nuttall AL, Scheibe F, et al. Sound-induced artifact in cochlear blood flow measurements using the laser Doppler flowmeter. Hear Res 1987;31:229-34. [PubMed]
- Dziennis S, Reif R, Zhi Z, et al. Effects of hypoxia on cochlear blood flow in mice evaluated using Doppler optical microangiography. J Biomed Opt 2012;17:106003. [PubMed]
- Reif R, Qin J, Shi L, et al. Monitoring hypoxia induced changes in cochlear blood flow and hemoglobin concentration using a combined dual-wavelength laser speckle contrast imaging and Doppler optical microangiography system. PLoS One 2012;7:e52041. [PubMed]
- Leitgeb R, Schmetterer L, Drexler W, et al. Real-time assessment of retinal blood flow with ultrafast acquisition by color Doppler Fourier domain optical coherence tomography. Opt Express 2003;11:3116-21. [PubMed]
- Makita S, Hong Y, Yamanari M, et al. Optical coherence angiography. Opt Express 2006;14:7821-40. [PubMed]
- Szkulmowska A, Szkulmowski M, Szlag D, et al. Three-dimensional quantitative imaging of retinal and choroidal blood flow velocity using joint Spectral and Time domain Optical Coherence Tomography. Opt Express 2009;17:10584-98. [PubMed]
- Tao YK, Davis AM, Izatt JA. Single-pass volumetric bidirectional blood flow imaging spectral domain optical coherence tomography using a modified Hilbert transform. Opt Express 2008;16:12350-61. [PubMed]
- Vakoc B, Yun S, de Boer J, et al. Phase-resolved optical frequency domain imaging. Opt Express 2005;13:5483-93. [PubMed]
- Wang RK. Optical microangiography: a label free 3D imaging technology to visualize and quantify blood circulations within tissue Beds in vivo. IEEE J Sel Top Quantum Electron 2010;16:545-54. [PubMed]
- Reif R, Wang RK. Label-free imaging of blood vessel morphology with capillary resolution using optical microangiography. Quant Imaging Med Surg 2012;2:207-12. [PubMed]
- Choudhury N, Chen F, Shi X, et al. Volumetric Imaging of Blood Flow within Cochlea in Gerbil in vivo. IEEE J Sel Top Quantum Electron 2009;PP:1-6.
- Subhash HM, Davila V, Sun H, et al. Volumetric in vivo imaging of intracochlear microstructures in mice by high-speed spectral domain optical coherence tomography. J Biomed Opt 2010;15:036024. [PubMed]
- Subhash HM, Davila V, Sun H, et al. Volumetric in vivo imaging of microvascular perfusion within the intact cochlea in mice using ultra-high sensitive optical microangiography. IEEE Trans Med Imaging 2011;30:224-30. [PubMed]
- Duvall AJ 3rd, Ward WD, Lauhala KE. Stria ultrastructure and vessel transport in acoustic trauma. Ann Otol Rhinol Laryngol 1974;83:498-514. [PubMed]
- Axelsson A, Vertes D, Miller J. Immediate noise effects on cochlear vasculature in the guinea pig. Acta Otolaryngol 1981;91:237-46. [PubMed]
- Dengerink H, Miller J, Axelsson A, et al. The recovery of vascular changes following brief noise exposure. Acta Otolaryngol 1985;100:19-25. [PubMed]
- Scheibe F, Haupt H, Nuttall AL, et al. Laser doppler measurements of cochlear blood flow during loud sound presentation. Eur Arch Otorhinolaryngol 1990;247:84-8. [PubMed]
- Perlman HB, Kimura R. Cochlear blood flow in acoustic trauma. Acta Otolaryngol 1962;54:99-110. [PubMed]
- Ryan AF, Axelsson A, Myers R, et al. Changes in cochlear blood flow during acoustic stimulation as determined by 14C-iodoantipyrine autoradiography. Acta Otolaryngol 1988;105:232-41. [PubMed]
- Prazma J, Vance SG, Bolster DE, et al. Cochlear blood flow. The effect of noise at 60 minutes’ exposure. Arch Otolaryngol Head Neck Surg 1987;113:36-9. [PubMed]
- Nuttall AL, Hultcrantz E, Lawrence M. Does loud sound influence the intracochlear oxygen tension? Hear Res 1981;5:285-93. [PubMed]
- Jero J, Tseng CJ, Mhatre AN, et al. A surgical approach appropriate for targeted cochlear gene therapy in the mouse. Hear Res 2001;151:106-14. [PubMed]
- Li P, Reif R, Zhi Z, et al. Phase-sensitive optical coherence tomography characterization of pulse-induced trabecular meshwork displacement in ex vivo nonhuman primate eyes. J Biomed Opt 2012;17:076026. [PubMed]
- Wang RK, An L. Doppler optical micro-angiography for volumetric imaging of vascular perfusion in vivo. Opt Express 2009;17:8926-40. [PubMed]
- Zhi Z, Cepurna W, Johnson E, et al. Volumetric and quantitative imaging of retinal blood flow in rats with optical microangiography. Biomed Opt Express 2011;2:579-91. [PubMed]
- Srinivasan VJ, Sakadzić S, Gorczynska I, et al. Quantitative cerebral blood flow with optical coherence tomography. Opt Express 2010;18:2477-94. [PubMed]
- Zhi Z, Yin X, Dziennis S, et al. Optical microangiography of retina and choroid and measurement of total retinal blood flow in mice. Biomed Opt Express 2012;3:2976-86. [PubMed]
- Iwagaki T, Suzuki T, Nakashima T. Development and regression of cochlear blood vessels in fetal and newborn mice. Hear Res 2000;145:75-81. [PubMed]
- Cantos R, Cole LK, Acampora D, et al. Patterning of the mammalian cochlea. Proc Natl Acad Sci U S A 2000;97:11707-13. [PubMed]
- Müller M, von Hünerbein K, Hoidis S, et al. A physiological place-frequency map of the cochlea in the CBA/J mouse. Hear Res 2005;202:63-73. [PubMed]


