
Neuroradiological findings in three cases of pontocerebellar hypoplasia type 9 due to AMPD2 mutation: typical MRI appearances and pearls for differential diagnosis
Introduction
Pontocerebellar hypoplasia type 9 (PCH9) is a rare autosomal recessive neurodegenerative disorder with prenatal onset caused by mutations in the adenosine monophosphate deaminase 2 (AMPD2) gene (OMIM# 615809) which is necessary for guanine nucleotide biosynthesis, protein translation and neurogenesis (1). Affected patients present with neurodevelopmental delay, truncal hypotonia, neonatal clonus and facial dysmorphism. Cerebral visual impairment, swallowing difficulties, spasticity and hypereflexia are always present (2-5). Neuroimaging features have been recently described and include pontine hypoplasia/atrophy, a figure of “8” of the midbrain on axial plane, cerebellar atrophy affecting the hemispheres more than the vermis, marked periventricular leukomalacia (PVL) and hypoplasia/absence of the corpus callosum (1-5).
The aim of this article is to report the neuroradiological findings in three new cases of PCH9 with AMPD2 mutation and provide a review of the literature on radiological appearances of other pontocerebellar hypoplasias (PCHs) with a specific focus on their differential diagnosis. With this short report, we intend to raise awareness among radiologists on the fairly typical neuroradiological findings in PCH9 and allow prompt diagnosis of this rare condition.
Case series
Clinical presentation and evolution
Patient 1
A 7-year-old female with a homozygous pathogenic variant of AMPD2 (both parents were heterozygous variants). She presented with severe global developmental delay and arrest of development in the first years of life. Subsequently, she developed spasticity in all four limbs, visual impairment, feeding difficulties that required gastrostomy, subluxation of hips and bilateral talipes.
Patient 2
Sister of patient 1; she presented at 4 years of age with strikingly similar symptoms and signs to her sister (developmental arrest, congenital talipes and spasticity).
Patient 3
A 2-year-old female heterozygous for a non-sense pathogenic variant of AMPD2 and for a missense variant in the same gene. She presented at 4 months of age with microcephaly, an evolving spastic-dystonic movement disorder, truncal hypotonia, intractable epilepsy and abnormal visual behavior.
Magnetic resonance imaging (MRI) findings
All patients underwent MRI of the brain: patient 1 at 9 months of age, patient 2 at 7 months of age and patient 3 at 18 months of age. MRI findings were very similar in all individuals: the cerebellum and the pons were hypoplastic with a “dragonfly” appearance (i.e., marked atrophy of the hemispheres and a relative sparing of the vermis), the midbrain in axial views had a figure of “8” appearance, the corpus callosum was severely hypoplastic and there were diffuse PVL and global reduction of the white matter bulk (resembling the pattern of PVL described in preterm infant but more extensive). Moreover, the basal ganglia and thalami were severely hypoplastic, in particular the globi pallidi.
Figure 1 shows the constellation of findings in the subjects.
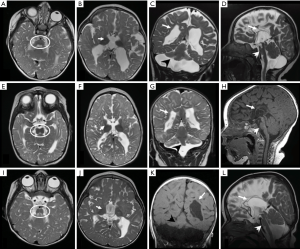
Informed consent was obtained from the parents of all patients included in the study.
Discussion
PCH9 is a rare autosomal recessive neurodegenerative disorders with prenatal onset presenting with severe neurodevelopmental delay, microcephaly, axonal neuropathy and epilepsy (1,2). We describe 3 patients with strikingly similar constellation of brain findings. Abnormalities on MRI were also comparable to those previously described in literature and include a characteristic combination of “dragonfly” PCH, a figure of “8” appearance of midbrain on axial plane, progressive microcephaly and marked global reduction of the cerebral white matter in keeping with PVL. As a consequence of the marked reduction of the cerebral white matter bulk, the corpus callosum was extremely thinned or absent (2-5). The figure of “8” shape of the midbrain probably results from a marked thinning of cortico-spinal tract, as shown by a previous DTI study (3).
Our cases and the scarce literature available confirm that the diagnosis of AMPD2 mutation/PCH9 can be done based on pathognomonic brain MRI findings.
Akizu et al. (4) reported 5 patients with a similar structural brain anomaly characterized by hypoplasia/atrophy of cerebellum and flattening of the ventral part of pons but also atrophy of cerebral cortex and hypoplasia of corpus callosum. In the cerebellum, relative sparing of the vermis, gave a characteristic dragonfly appearance. Marsh et al. (2) described 5 affected siblings with AMPD2 mutation all showing the same neuroradiological findings but with complete absence of the corpus callosum. One patient in this series had both prenatal (at 30 weeks gestation) and postnatal MRI scans suggesting a fetal onset with progressive loss of volume overtime. Interestingly our cases show preservation of the cingulate gyrus. Thus, we think that, given the embryological link between cingulate gyrus and corpus callosum, and the progressive, rather than absent, PVL in all PCH9, the callosum may be just extremely thinned in these patients.
To date, 17 variants have been associated with PCH9: ten missense, three nonsense, three frameshift and one splice site variant (1). The PCH9-associated AMPD2 pathogenic variants mainly localize within the catalytic AMPD2 domain (2,4), this gene encodes one of three known AMP deaminase homologs which is necessary for guanine nucleotide biosynthesis, protein translation and neurogenesis (4).
Radiological differential diagnosis with other PCH subtypes (Figure 2): a pattern-recognition approach
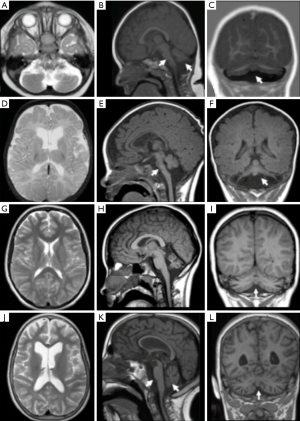
A total of 11 different subtypes of PCH have been described according to their genetic basis; they show a remarkable clinical variability and differ also in terms of neuroimaging findings. As a general rule, pontocerebellar involvement is a consistent feature on MRI, even if severe cerebellar symptoms are seldom described.
“Dragonfly” vs. “Butterfly” cerebellum
Based on coronal images, the cerebellar appearances can be distinguished into different groups: a dragonfly type, with relatively preserved vermis and atrophic cerebellar hemispheres and a butterfly type where hemispheres and vermis are both reduced in size to a similar extent. A typical example of dragonfly patterns is PCH2A due to TSEN54 mutation (6-8). On the other hand, PCH8 patients show profound PCH with a dragonfly appearance and no evidence of disease progression (7). In PCH1, due to EXOSC3 mutation, both dragonfly and butterfly patterns of cerebellar hemisphere involvement have been described, with the dragonfly pattern being the most frequent (9).
We have summarized the most relevant imaging features of the PCH subtypes in Table 1.
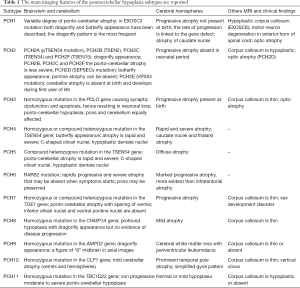
Full table
Specific MRI patterns
- CASK-related disorders, due to mutations or deletions of the CASK gene, are characterized by microcephaly and butterfly PCH, possible asymmetry of cerebellar hemispheres and preserved corpus callosum may be present (apart from PCH9) (10). These patients are predominantly females.
- Other dragonfly PCHs are PCH2D, caused by SEPSECs mutation (11), and PCH4. Additionally in PCH4 MRI shows C-shaped inferior cerebellar olives, due to their lack of folding, this is the reason why the disease was also known as olivoPCH (12). Moreover in PCH2D pontine hypoplasia can be absent (11) (i.e., only dragonfly cerebellum).
- Extreme thinning or even dysgenesis of corpus callosum may be present (together with PCH9) in PCH8, PCH1 subtype caused by EXOSC8 mutation, PCH2D, PCH10 and PCH11 (2,4,7,11,12). Thus, the size of corpus callous may range from normal (i.e., CASK) to very atrophic, and needs to be always evaluated when the suspect of PCH arises on images.
- Cortical malformation associated with marked vermian atrophy are described in PCHs due to mutations in RELN and VLDLR genes (7). Moreover in CASK-related disorders neocortical malformations, with a reduced gyral pattern, can also be observed (10).
- Diffuse T2/FLAIR hyperintensity of the cerebellar cortex in the context of PCH can suggest the diagnosis of congenital disorders of glycosylation type 1a (CDG-1a) (13), although this is not a very specific finding in our experience as it is described also in other mitochondrial diseases (coenzyme Q10 deficiency and complex I deficiency due to NUBPL mutations) and other neurodegenerative diseases (infantile neuroaxonal dystrophy and late infantile neuronal ceroid lipofuscinosis) (14).
- Evolution of the atrophy: cerebral atrophy can be present at birth, as in PCH3, or can occur later and progress over time, as in PCH1, PCH2, PCH6, and PCH7 (7,9,11,15). As a result, each PCH subtype has a highly variable disease course. For instance, the progression of symptoms and of atrophy, is more rapid and severe in PCH4, PCH5 and PCH6 whereas in PCH8 and PCH11 there is no evidence of disease progression (7). The term PCH can be misleading as it suggests a static process. Nevertheless, the PCH group includes non-progressive diseases (types 3 and 8) as well as prenatal onset degenerative disorders (types 1, 2, 4, 5, 6, and 7) (7) in which the term atrophy would be more appropriate.
- Ophthalmology complications in PCH may relate to optic atrophy, which is well described in PCH1 (16) as well as in PCH2D, PCH3 and CASK-related disorders (7,10,12).
- Measurements are more useful in cases of suspected hypoplastic cerebellum than in atrophy cases. Most of the normal values available in literatures refers to ratio with the supratentorial brain and seem to be also useful in evaluation of cerebellar enlargement (macrocerebellum) (17).
- Pseudo-PVL pattern can be found in patients with PCH7 as a marked reduction in the white matter bulk with severe ventriculomegaly. This pseudo-PVL pattern is usually associated with corpus callosum anomalies, and an equal involvement of vermis and hemispheres although with a higher degree of hypoplasia and atrophy (7). However, clinical features are strikingly different and the radiological features can help formulating this differential diagnosis.
Conclusions
PCH9 has typical imaging findings characterized by dragonfly cerebellar atrophy, PVL, extreme thinning of the corpus callosum and abnormal midbrain describing a figure of “8” in axial images. This constellation of findings may be considered pathognomonic and can be used to differentiate PCH9 from other PCH subtypes.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Marsh APL, Novarino G, Lockhart PJ, Leventer RJ. CUGC for pontocerebellar hypoplasia type 9 and spastic paraplegia-63. Eur J Hum Genet 2019;27:161-6. [Crossref] [PubMed]
- Marsh APL, Lukic V, Pope K, Bromhead C, Tankard R, Ryan MM, Yiu EM, Sim JC, Delatycki MB, Amor DJ, McGillivray G, Sherr EH, Bahlo M, Leventer RJ, Lockhart PJ. Complete callosal agenesis, pontocerebellar hypoplasia, and axonal neuropathy due to AMPD2 loss. Neurol Genet 2015;16;1:e16.
- Accogli A, Iacomino M, Pinto F, Orsini A, Vari MS, Selmi R, Torella A, Nigro V, Minetti C, Severino M, Striano P, Capra V, Zara F. Novel AMPD2 mutation in pontocerebellar hypoplasia, dysmorphisms, and teeth abnormalities. Neurol Genet 2017;3:e179. [Crossref] [PubMed]
- Akizu N, Cantagrel V, Schroth J, Cai N, Vaux K, McCloskey D, Naviaux RK, Van Vleet J, Fenstermaker AG, Silhavy JL, Scheliga JS, Toyama K, Morisaki H, Sonmez FM, Celep F, Oraby A, Zaki MS, Al-Baradie R, Faqeih EA, Saleh MA, Spencer E, Rosti RO, Scott E, Nickerson E, Gabriel S, Morisaki T, Holmes EW, Gleeson JG. AMPD2 regulates GTP synthesis and is mutated in a potentially treatable neurodegenerative brainstem disorder. Cell 2013;154:505-17. [Crossref] [PubMed]
- Marsh APL, Yap P, Tan T, Pope K, White SM, Chong B, Mcgillivray G, Boys A, Stephenson SE, Leventer RJ, Stark Z, Lockhart PJ. A novel AMPD2 mutation outside the AMP deaminase domain causes pontocerebellar hypoplasia type 9. Am J Med Genet A 2017;173:820-23. [Crossref] [PubMed]
- Namavar Y, Barth PG, Kasher PR, van Ruissen F, Brockmann K, Bernert G, Writzl K, Ventura K, Cheng EY, Ferriero DM, Basel-Vanagaite L, Eggens VR, Krägeloh-Mann I, De Meirleir L, King M, Graham JM Jr, von Moers A, Knoers N, Sztriha L, Korinthenberg R, Consortium PCH, Dobyns WB, Baas F, Poll-The BT. Clinical, neuroradiological and genetic findings in pontocerebellar hypoplasia. Brain 2011;134:143-56. [Crossref] [PubMed]
- van Dijk T, Baas F, Barth PG, Poll-The BT. What’s new in pontocerebellar hypoplasia? An update on genes and subtypes. Orphanet J Rare Dis 2018;13:92. [Crossref] [PubMed]
- Feinstein M, Flusser H, Lerman-Sagie T, Ben-Zeev B, Lev D, Agamy O, Cohen I, Kadir R, Sivan S, Leshinsky-Silver E, Markus B, Birk OS. VPS53 mutations cause progressive cerebello-cerebral atrophy type 2 J Med Genet 2014;51:303-8. [PCCA2]. [Crossref] [PubMed]
- Eggens VR, Barth PG, Niermeijer JMF, Berg JN, Darin N, Dixit A, Fluss J, Foulds N, Fowler D, Hortobágyi T, Jacques T, King MD, Makrythanasis P, Máté A, Nicoll JA, O'Rourke D, Price S, Williams AN, Wilson L, Suri M, Sztriha L, Dijns-de Wissel MB, van Meegen MT, van Ruissen F, Aronica E, Troost D, Majoie CB, Marquering HA, Poll-Thé BT, Baas F. EXOSC3 mutations in pontocerebellar hypoplasia type 1: novel mutations and genotype-phenotype correlations. Orphanet J Rare Dis 2014;9:23. [Crossref] [PubMed]
- Burglen L, Chantot-Bastaraud S, Garel C, Milh M, Touraine R, Zanni G, Petit F, Afenjar A, Goizet C, Barresi S, Coussement A, Ioos C, Lazaro L, Joriot S, Desguerre I, Lacombe D, des Portes V, Bertini E, Siffroi JP, de Villemeur TB, Rodriguez D. Spectrum of pontocerebellar hypoplasia in 13 girls and boys with CASK mutations: confirmation of a recognizable phenotype and first description of a male mosaic patient. Orphanet J Rare Dis 2012;7:18. [Crossref] [PubMed]
- Pavlidou E, Salpietro V, Phadke R, Hargreaves IP, Batten L, McElreavy K, Pitt M, Mankad K, Wilson C, Cutrupi MC, Ruggieri M, McCormick D, Saggar A, Kinali M. Pontocerebellar hypoplasia type 2D and optic nerve atrophy further expand the spectrum associated with selenoprotein biosynthesis deficiency. Eur J Paediatr Neurol 2016;20:483-8. [Crossref] [PubMed]
- Namavar Y, Barth PG, Poll-The BT, Baas F. Classification, diagnosis and potential mechanisms in pontocerebellar hypoplasia. Orphanet J Rare Dis 2011;6:50. [Crossref] [PubMed]
- Feraco P, Mirabelli-Badenier M, Severino M, Alpigiani MG, Di Rocco M, Biancheri R, Rossi A. The shrunken, bright cerebellum: a characteristic MRI finding in congenital disorders of glycosylation type 1a. AJNR Am J Neuroradiol 2012;33:2062-7. [Crossref] [PubMed]
- Anderson C, Davies JH, Lamont L, Foulds N. Early pontocerebellar hypoplasia with vanishing testes: A new syndrome? Am J Med Genet A 2011;155A:667-72. [Crossref] [PubMed]
- Ivanov I, Atkinson D, Litvinenko I, Angelova L, Andonova S, Mumdjiev H, Pacheva I, Panova M, Yordanova R, Belovejdov V, Petrova A, Bosheva M, Shmilev T, Savov A, Jordanova A. Pontocerebellar hypoplasia type 1 for the neuropediatrician: Genotype-phenotype correlations and diagnostic guidelines based on new cases and overview of the literature. Eur J Paediatr Neurol 2018;22:674-81. [Crossref] [PubMed]
- D’Arco F, Ugga L, Caranci F, Riccio MP, Figliuolo C, Mankad K, D’Amico A. Quant Imaging Med Surg 2016;6:496-503. [Crossref] [PubMed]
- Poretti A, Wolf NI, Boltshauser E. Differential diagnosis of cerebellar atrophy in childhood. Eur J Paediatr Neurol 2008;12:155-67. [Crossref] [PubMed]


