
Tuberculosis mediastinal fibrosis misdiagnosed as chronic bronchitis for 10 years: a case report
Background
Mediastinal fibrosis (MF, or fibrosing mediastinitis) is a rare condition characterized by rapid increase of fibrous tissues in the mediastinum and is often associated with granulomatous diseases such as histoplasmosis, tuberculosis, sarcoidosis, and other fibroinflammatory and autoimmune diseases (1-3). It can cause compression and obliteration of vital mediastinal structures, e.g., trachea, esophagus, and great vessels (4,5). Given that the disease is rare and its clinical symptoms are similar to other respiratory diseases, misdiagnosis is common. Herein, we describe a patient with diffuse MF who had been misdiagnosed as chronic bronchitis and pulmonary hypertension for the past 10 years.
Case presentation
Clinical history
A 69-year-old woman was admitted to our hospital with a 10-year history of productive cough and hypertension, and shortness of breath and reduced activity tolerance for 2 months. This patient, a housewife and non-smoker, was hospitalized several times during the past ten years because of recurrent cough with sputum and opportunistic infections without fever, chest pain, fatigue, night sweats, and weight loss. She had no history of exposure to dust or other contact allergens. She was suspected of chronic bronchitis and intermittently treated with antibiotics with no efficacy for nearly 10 years. As her symptoms progressed, shortness of breath and reduced activity tolerance had been aggravating over previous 2 months. All vital signs at presentation were normal (temperature: 37.6 °C, heart rate: 97/min, blood pressure: 155/95 mmHg, saturation: 95%). Clinical physical examination revealed a lip purpura and reduced breath sound in the right lung.
Laboratory tests
Blood test results were as follows: blood cell count and liver and kidney function normal. C-reactive protein (CRP) 26 mg/L, erythrocyte sedimentation rate (ESR) 83 mm/h, N terminal pro-B-type natriuretic peptide (NT-proBNP) 288 ng/L. Common neoplastic markers [carcinoembryonic antigen (CEA), neuron-specific enolase (NSE), alpha fetoprotein (AFP), cancer antigen 125 (CA125), cancer antigen 153 (CA153), serum squamous cell carcinoma antigen 19 (SCC-19)] all negative. Autoantibodies [anti-nuclear antibody (ANA), antigenic peptide antibody spectrum (anti-ENA) antibodies, antineutrophil cytoplasmic antibody (ANCA)] all negative. Humoral and cellular immunity markers (C3, C4, IgG4, T lymphocyte subset analysis) all negative. Serum angiotensin converting enzyme (SACE) 71.5 U/L.
Blood gas analysis results: potential of hydrogen (pH) 7.36, PaO2 75 mmHg, PaCO2 35 mmHg. Tuberculosis immunology index: antituberculosis antibody LAM (+), IgG (−), 16 KD (−), 38 KD (−); T cell detection of TB infection (T-spot): reactive (ESAT-6 70, CFP-10 30); PPD (−). Electrocardiography: Sinus rhythm, T-wave inversion in V3 and V4. Echocardiography: mild mitral and tricuspid regurgitation, a mean pulmonary artery (PA) pressure of 79 mmHg. Spirometry: forced expiratory volume in the first second (FEV1): 1.72 (56.8% predicted), forced vital capacity (FVC): 2.09 (91.5% predicted), FEV1/FVC: 51%, Moderate obstructive cardiovascular disability.
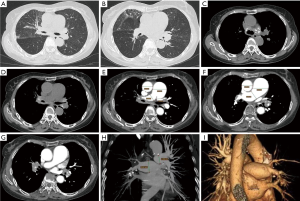
CT image findings
The high-resolution non-enhanced CT images revealed extensive bronchovascular bundle thickening, interlobular septum thickening in the right upper lobe, and slight ground-glass patchy shadow in the subpleural region of the upper lobe of right lung. The right main bronchial wall was thickened with the lumen irregularly narrowed. Multiple soft tissue density lesions with calcifications on bilateral hilar and mediastinum were noted, and compressed and wrapped the pulmonary artery branches, resulting in the their lumens narrowed severely. Partial pulmonary artery branch lumen showed close to occlusion. On the other hand, the main pulmonary artery trunk widened significantly. The widest section diameter was 4.31 cm, the ratio of the transverse diameter to the aorta was greater than 1.0. (4.31/3.21=1.34) (Figure 1A,B,C,D,E,F,G). With three-dimensional image post-processing, the multiple planar reconstruction (MPR) and volume rendering (VR) images demonstrated the appearance of thinned pulmonary artery branches (Figure 1H,I).
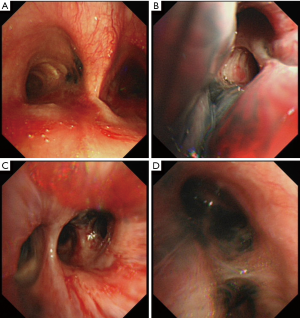
Bronchoscopy and bronchoalveolar lavage (BAL) fluid test
Bronchoscopy detected widely distorted bronchi with multiple incomplete stenoses with hypervascularity and pigmentation (Figure 2). BAL showed benign bronchial cells, abundant macrophages, few multinucleated giant cells, polymorphonuclear leukocytes, and lymphocytes. Cultures for bacteria, acid-fast bacilli, and fungi were all negative during initial presentation. Cultures of BAL fluid for tuberculosis showed positive after 2 months and sensitive to the first-line anti-tuberculosis drugs.
Diagnosis and treatment
Based on the aforementioned clinical, radiological, and bronchoscopic findings, and after ruling out other possibilities, we concluded that the patient had MF, with the potential pathogenetic mechanisms being tuberculosis. Treatment with isoniazid (300 mg/day), rifampin (600 mg/day), pyrazinamide (750 mg/day) and ethambutol (500 mg/day) was started. At the same time, she received immunosuppressive therapy with prednisolone (30 mg/day).
Discussion
MF is a rare disease characterized by fibrous proliferation in the mediastinum (1-3). Accurate epidemiological data is lacking given that most published articles are case reports (6-9). The most common symptoms include truncal pain, respiratory symptoms, and other systemic inflammatory symptoms such as cough, dyspnea, hemoptysis, pleuritic chest pain, or pulmonary infections (10-15). With a lack of specific clinical manifestations, misdiagnosis is common in the early stage. Our patient was diagnosed as chronic bronchitis for 10 years, resulting in prolonged illness. The pathogenesis of MF is unclear; however, numerous case reports have described its association with fibro-inflammatory and autoimmune disorders, such as ANCA-associated vasculitis, Behçet’s disease, and IgG4-related disease (IgG4-RD) (2,3). A number of triggers with pathogenic microbial infections have been associated with MF, particularly Histoplasma capsulatum infections. Other infections include tuberculosis, nocardiosis, aspergillosis, cryptococcosis, and atypical mycobacterial infections (1). In our case, we diagnosed the potential pathogenetic mechanisms being tuberculosis, as the cultures of BAL fluid for tuberculosis was positive. Infecting mycobacteria may share antigens with human tissue, thus partially accounting for the high frequency of autoantibodies in patients with mycobacterial infections.
For MF, CT images typically show infiltrative soft tissue lesions that obliterates normal mediastinal fat spaces, and wraps or invades adjacent structures, with mediastinal involvement and calcification and bilateral hilar calcified lymph nodes (16,17). The contrast-enhanced CT of our patient showed ill-defined infiltrative bilateral hilar and mediastinum soft tissue density masses with calcifications, suggesting the possibility of previous tuberculosis infection. The mediastinal and bilateral hila soft-tissue resulted in deformed stenosis of segmental bronchus and pulmonary arteries by invading and compressing bronchial and vascular walls. Widening of the pulmonary artery trunk was consistent with the pulmonary artery echocardiography and thickened interlobular septa in the peripheral region of right upper lobe due to interstitial effusion. MF can progress slowly and cause respiratory symptoms such as asthma. The clinical course of our patients appeared to be more benign and less progressive. We should emphasize the importance of enhanced CT examination because it can help find the range and extent of the MF lesions and avoid delays in confirmed diagnosis. This is one of reasons for the delayed diagnosis of our case. There has been shown a relationship between dark anthracotic pigmentation in the bronchial mucosa and tuberculosis. These endoscopic features seen in this patient, suggesting MF caused by tuberculosis infection.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Liu T, Gao L, Xie S, Sun H, Liu M, Zhai Z. Clinical and imaging spectrum of tuberculosis-associated fibrosing mediastinitis. Clin Respir J 2018;12:1974-80. [Crossref] [PubMed]
- Rossi GM, Emmi G, Corradi D, Urban ML, Maritati F, Landini F, Galli P, Palmisano A, Vaglio A. Idiopathic Mediastinal Fibrosis: a Systemic Immune-Mediated Disorder. A Case Series and a Review of the Literature. Clin Rev Allergy Immunol 2017;52:446-59. [Crossref] [PubMed]
- Takanashi S, Akiyama M, Suzuki K, Otomo K, Takeuchi T. IgG4-related fibrosing mediastinitis diagnosed with computed tomography-guided percutaneous needle biopsy: Two case reports and a review of the literature. Medicine (Baltimore) 2018;97:e10935. [Crossref] [PubMed]
- Cimenoglu B, Ozkan B, Basaran M, Toker A. Pulmonary Arterial Bypass Surgery for Fibrosing Mediastinitis Causing Severe Pulmonary Hypertension. Ann Thorac Surg 2019;107:411-3. [Crossref] [PubMed]
- Goldbach AR, Pascarella S, Dadpravarar S. Fibrosing Mediastinitis: a Rare Cause of Unilateral Absent Lung Perfusion on a V/Q Scan. Nucl Med Mol Imaging. 2018;52:401-4. [Crossref] [PubMed]
- Hevroni A, Springer C, Wasser O, Avital A, Koplewitz BZ. Recurrent Pneumonia due to Fibrosing Mediastinitis in a Teenage Girl: A Case Report with Long-Term Follow-Up. Case Rep Pediatr 2018;2018:3246929. [Crossref] [PubMed]
- Tan R, Martires J, Kamangar N. Tuberculosis-associated Fibrosing Mediastinitis: Case Report and Literature Review. J Clin Imaging Sci 2016;6:32. [Crossref] [PubMed]
- Kern R, Peikert T, Edell E, Mullon J, Midthun D, Utz J, Maldonado F. Bronchoscopic Management of Airway Compression due to Fibrosing Mediastinitis. Ann Am Thorac Soc 2017;14:1353-5. [Crossref] [PubMed]
- Li Y, Meng X, Wang Y, Yang Y, Lu X. Fibrosing mediastinitis with pulmonary hypertension as a complication of pulmonary vein stenosis: A case report and review of the literature. Medicine (Baltimore) 2018;97:e9694. [Crossref] [PubMed]
- Mirsadraee M, Saffari A, Sarafraz Yazdi M, Meshkat M. Frequency of tuberculosis in anthracosis of the lung: A systematic review. Arch Iran Med 2013;16:661-4. [PubMed]
- Han FF, Yang TY, Song L, Zhang Y, Li HM, Guan WB, Liu Q, Guo XJ. Clinical and pathological features and imaging manifestations of bronchial anthracofibrosis: the findings in 15 patients. Chin Med J 2013;126:2641-6. [PubMed]
- Kim HJ, Kim SD, Shin DW, Bae SH, Kim AL, Kim JN, Jung SW, Lee BK, Kim YJ. Relationship between bronchial anthracofibrosis and endobronchial tuberculosis. Korean J Intern Med 2013;28:330-8. [Crossref] [PubMed]
- Hu Y, Qiu JX, Liao JP, Zhang H, Jin Z, Wang GF. Clinical Manifestations of Fibrosing Mediastinitis in Chinese Patients. Chin Med J 2016;129:2697-702. [Crossref] [PubMed]
- Volkova KI, Kokosov AN, Malukalov BN, Brazhenko NA. Post-tuberculous fibrous mediastinitis: a report of a case followed-up for many years. Klin Med (Mosk) 2003;81:63-6. [PubMed]
- Wu Z, Jarvis H, Howard LS, Wright C, Kon OM. Post-tuberculous fibrosing mediastinitis: a review of the literature. BMJ Open Respir Res 2017;4:e000174. [Crossref] [PubMed]
- Atasoy C, Fitoz S, Erguvan B, Akyar S. Tuberculous fibrosing mediastinitis: CT and MRI findings. J Thorac Imaging 2001;16:191-3. [Crossref] [PubMed]
- McNeeley MF, Chung JH, Bhalla S, Godwin JD. Imaging of granulomatous fibrosing mediastinitis. AJR Am J Roentgenol 2012;199:319-27. [Crossref] [PubMed]


